DEPARTMENT OF HEALTH AND SOCIAL SERVICES
Division of Medicaid and Medical Assistance
PROPOSED
PUBLIC NOTICE
Pharmaceutical Services – Reimbursement of Covered Outpatient Drugs
In compliance with the State's Administrative Procedures Act (APA - Title 29, Chapter 101 of the Delaware Code), 42 CFR §447.205, and under the authority of Title 31 of the Delaware Code, Chapter 5, Section 512, Delaware Health and Social Services (DHSS) / Division of Medicaid and Medical Assistance (DMMA) is proposing to amend the Title XIX Medicaid State Plan regarding Pharmaceutical Services, specifically, to clarify reimbursement methodology for covered outpatient drugs.
Any person who wishes to make written suggestions, compilations of data, testimony, briefs or other written materials concerning the proposed new regulations must submit same to, Planning, Policy and Quality Unit, Division of Medicaid and Medical Assistance, 1901 North DuPont Highway, P.O. Box 906, New Castle, Delaware 19720-0906, by email to Kimberly.Xavier@state.de.us, or by fax to 302-255-4425 by December 1, 2016. Please identify in the subject line: Pharmaceutical Services - Reimbursement of Covered Out-Patient Drugs
The action concerning the determination of whether to adopt the proposed regulation will be based upon the results of Department and Division staff analysis and the consideration of the comments and written materials filed by other interested persons.
SUMMARY OF PROPOSAL
The purpose of this notice is to advise the public that Delaware Health and Social Services (DHSS)/Division of Medicaid and Medical Assistance (DMMA) is proposing to amend the Title XIX Medicaid State Plan regarding Pharmaceutical Services, specifically, to clarify reimbursement methodology for covered outpatient drugs.
Statutory Authority
Background
Under the Medicaid program, States may provide coverage of outpatient drugs as an optional service under section 1905(a)(12) of the Social Security Act (the Act). Section 1903(a) of the Act provides for Federal financial participation (FFP) in State expenditures for these drugs. States generally reimburse pharmacies for prescribed covered outpatient drugs dispensed to Medicaid beneficiaries based on a two-part formula consisting of the ingredient cost of a drug and a professional dispensing fee. States have flexibility to determine reimbursement amounts, consistent with applicable statutory and regulatory requirements. These reimbursement amounts are subject to review and approval by the Centers for Medicare & Medicaid Services (CMS) through the State Plan Approval (SPA) process.
On February 1, 2016 CMS published the Covered Outpatient Drug Rule. This rule became final on April 1, 2016 and implements provisions of the Patient Protection and Affordable Care Act of 2010, as amended by the Health Care and Education Reconciliation Act of 2010 (collectively referred to as the Affordable Care Act) pertaining to Medicaid reimbursement for covered outpatient drugs (CODs). The regulations direct the Medicaid programs to reimburse all outpatient covered drugs based on the actual acquisition cost of the medication and the professional dispensing fee if applicable.
The Division of Medicaid & Medical Assistance (DMMA) has been applying an Actual Acquisition Cost (AAC) plus a professional dispensing fee since April 1, 2016 for all dispensed products, as well as for medications that are administered in a clinical setting. Medications can be purchased through different avenues depending on the type of entity purchasing the drugs. Prices can be published using multiple methods. DMMA will no longer be using the drug file that list Average Wholesale Prices. The new drug file will contain the Wholesale Acquisition Cost. Additionally, CMS has requested that all possible sources of drugs have a corresponding definition for reimbursement. Drugs reimbursed when administered either in a clinic or physician's office are submitted using a procedure code. These codes have been manually reviewed to establish an acquisition cost for any provider. The SPA is documenting the steps that are taken to develop those reimbursement levels.
Summary of Proposal
Purpose
To add language to the Medicaid State plan to clarify the reimbursement methodology for covered outpatient drugs.
Summary of Proposed Changes
Effective for services provided on and after January 1, 2017 Delaware Health and Social Services/Division of Medicaid and Medical Assistance (DHSS/DMMA) proposes to amend Attachment 4.19-B Page 14 and Page 14a to clarify the reimbursement methodology for all outpatient medications for the DMMA beneficiaries, by defining the Actual Acquisition Cost Methodology used.
Public Notice
In accordance with the federal public notice requirements established at Section 1902(a)(13)(A) of the Social Security Act and 42 CFR 447.205 and the state public notice requirements of Title 29, Chapter 101 of the Delaware Code, Delaware Health and Social Services (DHSS)/Division of Medicaid and Medical Assistance (DMMA) gives public notice and provides an open comment period for thirty (30) days to allow all stakeholders an opportunity to provide input to the methods and standards governing payment methodology for pharmaceutical services. Comments must be received by 4:30 p.m. on December 1, 2016.
CMS Review and Approval
The provisions of this draft state plan amendment (SPA) are subject to the Centers for Medicare and Medicaid Services (CMS) review and approval. The draft SPA page(s) may undergo further revisions before and after submittal to CMS based upon public comment and/or CMS feedback. The final version may be subject to significant change.
Provider Manual Update
Also, upon CMS approval, the applicable Delaware Medical Assistance Program (DMAP) Provider Policy Specific Manuals will be updated. Manual updates, revised pages or additions to the provider manual are issued, as required, for new policy, policy clarification, and/or revisions to the DMAP program. Provider billing guidelines or instructions to incorporate any new requirement may also be issued. A newsletter system is utilized to distribute new or revised manual material and to provide any other pertinent information regarding manual updates.
Fiscal Impact
The proposed amendment is being implemented to clarify current practices attested to by DMAP pharmacy providers. Therefore, there is no impact on the General Fund.
DMMA PROPOSED REGULATION #16-023a
REVISED
ATTACHMENT 4.19-B
Page 14
STATE PLAN UNDER TITLE XIX OF THE SOCIAL SECURITY ACT
STATE: DELAWARE
METHODS AND STANDARDS FOR ESTABLISHING PAYMENT RATES – OTHER TYPES OF CARE
REIMBURSEMENT FOR PHARMACEUTICALS
Overview
The Delaware Medical Assistance Program (DMAP) will reimburse pharmaceuticals using the lower of:
Methodology for establishing AAC is provided in the table on page Attachment 4.19-B Page 14a.
Entities that qualify for special purchasing under Section 602 of the Veterans Health Care Act of 1992, and entities exempt from the Robinson-Patman Price Discrimination Act of 1936 must charge the DMAP no more than their actual acquisition cost (AAC) plus a professional dispensing fee. The AAC must be supported by invoice and payment documentation.
Entities that purchase Section 340B of the Public Health Service Act products must request to use these drugs for all DMAP patients, including Medicaid fee-for-service patients and for patients whose care is covered by Medicaid Managed Care Organizations.
Professional Dispensing Fee
The professional dispensing fee rate is ten dollars ($10.00). There is one-time professional fee per thirty (30)-day period unless the class of drugs is routinely prescribed for a limited number of days.
Definitions
Delaware Maximum Allowable Cost (DMAC) - a maximum price set for reimbursement:
Any willing provider can dispense the product.
DMMA PROPOSED REGULATION #16-023b
REVISED
ATTACHMENT 4.19-B
Page 14a
STATE PLAN UNDER TITLE XIX OF THE SOCIAL SECURITY ACT
STATE: DELAWARE
METHODS AND STANDARDS FOR ESTABLISHING PAYMENT RATES – OTHER TYPES OF CARE
REIMBURSEMENT FOR PHARMACEUTICALS
Federal Upper Limit (FUL) - The FUL is a federally defined price and constitutes the upper limit of reimbursement where a DMAC limit does not exist.
Non-Traditional Pharmacy - long term care and specialty pharmacies.
Traditional Pharmacy - retail independent and retail chain pharmacies.
Reimbursement Policy:
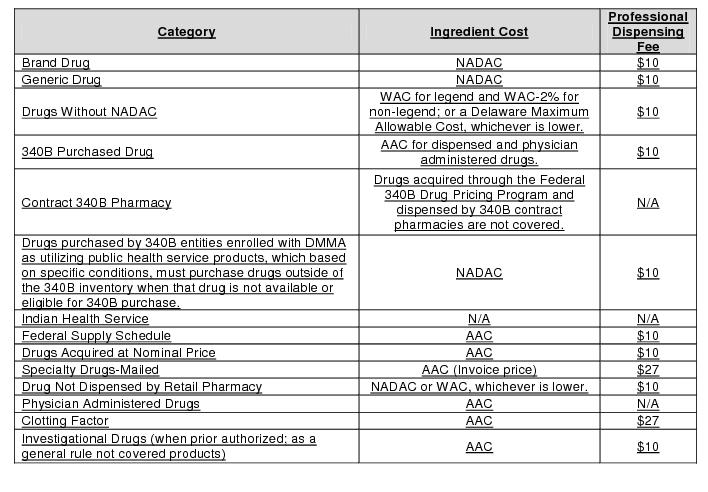
Exceptions:







