DEPARTMENT OF HEALTH AND SOCIAL SERVICES
Division Of Public Health
Authority On Radiation Protection
FINAL
ORDER
4465 Delaware Radiation Control Regulations
NATURE OF THE PROCEEDINGS:
Delaware Health and Social Services ("DHSS") initiated proceedings to amend five sets of the State of Delaware Regulations Governing Radiation Control. The DHSS proceedings to amend regulations were initiated pursuant to 29 Del.C. §101 and authority as prescribed by 16 Del.C. §7405. The five sets amended are shown in the table below.
|
DE Admin. Code No.
|
Current Delaware Citation
|
Title
|
|
4482
|
Part C
|
Licensing of Radioactive Material
|
|
4485
|
Part F
|
Use of Diagnostic X-Rays in the Healing Arts
|
|
4486
|
Part G
|
Use of Radionuclides in the Healing Arts
|
|
4490
|
Part K
|
Compliance Procedures
|
|
4492
|
Part X
|
Therapeutic Radiation Machines
|
On March 1, 2013 (Volume 16, Issue 9), DHSS published in the Delaware Register of Regulations its notice of proposed regulations, pursuant to 29 Del.C. §10115. It requested that written materials and suggestions from the public concerning the proposed regulations be delivered to DHSS by April 8, 2013, or be presented at a public hearing on March 22, 2013, after which time the DHSS would review information, factual evidence and public comment to the said proposed regulations.
Written comments were received during the public comment period and evaluated. The results of that evaluation are summarized in the accompanying "Summary of Evidence."
SUMMARY OF EVIDENCE
In accordance with Delaware Law, public notices regarding proposed Department of Health and Social Services (DHSS) Regulations Governing Radiation Control were published in the Delaware State News, the News Journal and the Delaware Register of Regulations.
Written comments were received on the proposed regulations during the public comment period (March 1, 2013 through April 8, 2013). Entities offering comments included:
Mr. Reed W. Best, Director of Regulatory and Quality Affairs, Aribex, Inc.
Public comments and the DHSS (Agency) responses are as follows:
Aribex is the developer and manufacturer of hand-held x-ray devices that are sold throughout the United States and in many other nations around the world for use in the dental, veterinarian, and forensic medicine. Currently Aribex distributes these units under the names of the NOMAD, NOMAD Pro, and the NOMAD eXaminer. All of these devices are manufacturer at the Aribex facilities in Orem, Utah. As a supplier of these hand-held x-ray units, the repeal of the current Part F and the addition of DE Admin Code No. 4485, Use of Diagnostic X-Rays in the Healing Arts are of direct importance to Aribex.
Aribex feels that the provisions in the proposed regulations are prudent and appropriate for the generic class of hand-held x-ray units. However, in order to reduce the administrative workload on both the staff of the Office of Radiation Control (ORC) and the regulated community, we would suggest that provisions be made either in the regulations or in the policies/procedures of the ORC to allow changes in these requirements based upon a demonstration by the manufacturer of a device that its device(s) are safe and efficacious.
California has issued Exemption to California Code of Regulations dated March 13, 2013, (copy is attached) which uses this approach:
“…exemption is made for users of the Aribex Nomad, Aribex Nomad Pro, and Aribex Nomad eXaminer, based on seven years of exposure data. Users of these portable hand-held X-ray systems are not required to comply with the personnel monitoring requirement found in condition 3. The personnel monitoring requirements may be reevaluated for other units after sufficient historical exposure data has been obtained and submitted, by the manufacturer, to the Department for review.”
Attached is a comparison of the requirements in the proposed regulations and what we suggest are appropriate requirements for the NOMAD devices. Specifically the differences are that the users of the NOMAD devices should not be required to use the device on a stand, should not be required to wear a leaded apron when operating the NOMAD, and should not be required to provide personnel monitoring devices for operators of the NOMAD.
These requirements, which are not required for operators of conventional wall mounted x-ray units, were put in place out of a need for an abundance of caution when the NOMAD was a new and unfamiliar device. Now with over seven years of clinical usage and over 11,000 NOMAD units in use across the United States, the NOMAD is no longer new or unfamiliar, but rather a proven safe and efficacious device. Therefore, Aribex requests that you reconsider these requirements for operators of the NOMAD.
The requirement for personnel monitoring in Delaware regulations applies to persons who may receive 10% or more of the annual radiation dose limit. In the numerous studies of operator doses associated with the use of the NOMAD, no study of radiation doses has ever shown anywhere near this level of exposure for operators of the NOMAD.
I believe that the staff of ORC has been provided with copies of many of these studies, but if you have not I will be happy to see that they are sent to you for your review. I have attached copies of two peer-reviewed studies on radiation doses to operators of the NOMAD published in 2012 in case you have not seen them. The first paper was published in Health Physics in February 2012 and the second was published in the Health Physics Society journal Operational Radiation Safety in August 2012. These studies, like all the previous studies, demonstrate that the radiation doses to operators of the NOMAD are extremely small and well below the 10% limit requiring personnel monitoring.
Aribex shares your concern that while the NOMAD has repeatedly been shown to be a safe and efficacious hand-held x-ray unit, there are other hand-held x-ray units available to users in Delaware that have not been designed and manufactured with the safety and radiation protection features inherent in the NOMAD. To our knowledge these units have not under gone the extensive radiation safety testing that the NOMAD has undergone.
The Washington radiation control program tested four different hand-held x-ray units including the NOMAD and found markedly different potential radiation exposures between the devices. Attached is a copy of the data from that study. Based on this study the State of Washington has adopted regulations that address the different levels of potential radiation exposure to the operators associated with various devices. A copy of the current Washington regulations that base the level of required additional protective devices based on potential radiation exposure to the operator is also attached. Delaware may want to consider providing similar criteria in its regulations or implementation guidance.
If Aribex can help the State of Delaware in its process of adopting its new regulations, please let me know.
Thank you for your time and consideration of our comments and suggestions.
Agency Response: The Agency appreciates and acknowledges your comment but respectfully will not adopt your recommendation, as the proposed regulation allows permitted facilities to utilize a conditional variance application process if they wish to use hand-held x-ray devices in the healing arts. In Delaware, permitted facilities have standing to request exemption or variance for utilization of any device approved by the FDA that they see as necessary in their practice, and the state inspects such facilities to assess operational safety and health protection regulatory compliance. The state has historically relied upon, and will continue to rely upon the federal Food & Drug Administration (FDA) to approve radiation-emitting manufactured devices for sale and distribution in the United States.
Verifying documents are attached to the Hearing Officer’s record. The regulation has been approved by the Delaware Attorney General’s office and the Cabinet Secretary of DHSS.
FINDINGS OF FACT:
Based on public comments received, non-substantive changes were made to the proposed regulations. The Department finds that the proposed regulations, as set forth in the attached copy should be adopted in the best interest of the general public of the State of Delaware.
THEREFORE, IT IS ORDERED, that the proposed State of Delaware Regulations Governing Radiation Control are adopted and shall become effective June 11, 2013, after publication of the final regulation in the Delaware Register of Regulations.
Rita M. Landgraf, Secretary
This part provides for the licensing of radioactive material, for purposes of protecting the public health and safety. No person shall receive, possess, use, transfer, sell, own or acquire radioactive material except as authorized in a specific or general license per the U.S. Nuclear Regulatory Commission (NRC), in accordance with Title 10 – Code of Federal Regulations. Primary radioactive material licensing and enforcement authority was transferred to the NRC in 2007, pursuant to the Federal Energy Policy Act of 2005. However, radioactive material facilities must be registered with the State of Delaware in accordance with 4481/Part B of these regulations.
Part F
This Part establishes requirements, for which a registrant is responsible, for use of diagnostic x-ray equipment by, or under the supervision of, an individual authorized by and licensed in accordance with State statutes to engage in the healing arts or veterinary medicine. The provisions of this Part are in addition to, and not in substitution for, other applicable provisions of Parts A, B, D, J and K of the regulations. Some registrants may also be subject to the requirements of Parts I and X of the regulations.
As used in this Part, the following definitions apply:
"Accessible surface" means the external surface of the enclosure or housing of the radiation producing machine as provided by the manufacturer.
"Accessory component" means:
(1) A component used with diagnostic x-ray systems, such as a cradle or film changer, that is not necessary for the compliance of the system with applicable provisions of this Part but which requires an initial determination of compatibility with the system; or
(2) A component necessary for compliance of the system with applicable provisions of this Part but which may be interchanged with similar compatible components without affecting the system’s compliance, such as one of a set of interchangeable beam-limiting devices; or
(3) A component compatible with all x-ray systems with which it may be used and that does not require compatibility or installation instructions, such as a tabletop cassette holder.
"Air kerma" means kerma in air (see definition of Kerma).
"Air kerma rate (AKR)" means the air kerma per unit time.
"Aluminum equivalent" means the thickness of type 1100 aluminum alloy (The nominal chemical composition of type 1100 aluminum is 99.00 percent minimum aluminum, 0.12 percent copper.) affording the same attenuation, under specified conditions, as the material in question.
"Articulated joint" means a joint between two separate sections of a tabletop which joint provides the capacity of one of the sections to pivot on the line segment along which the sections join.
"Assembler" means any person engaged in the business of assembling, replacing, or installing one or more components into an x-ray system or subsystem. The term includes the owner of an x-ray system or his or her employee or agent who assembles components into an x-ray system that is subsequently used to provide professional or commercial services.
"Attenuation block" means a block or stack, having dimensions 20 centimeters by 20 centimeters by 3.8 centimeters, of type 1100 aluminum alloy (The nominal chemical composition of type 1100 aluminum is 99.00 percent minimum aluminum, 0.12 percent copper.) or other materials having equivalent attenuation.
"Automatic exposure control (AEC)" means a device which automatically controls one or more technique factors in order to obtain at a preselected location(s) a required quantity of radiation (Includes devices such as phototimers and ion chambers).
"Automatic exposure rate control (AERC)" means a device which automatically controls one or more technique factors in order to obtain, at a preselected location(s), a required quantity of radiation per unit time.
"Barrier" (See "Protective barrier").
"Beam axis" means a line from the source through the centers of the x-ray fields.
"Beam-limiting device" means a device which provides a means to restrict the dimensions of the x- ray field.
"Bone densitometry system" means a medical device which uses electronically-produced ionizing radiation to determine the density of bone structures of human patients.
"C-arm fluoroscope" means a fluoroscopic x-ray system in which the image receptor and the x-ray tube housing assembly are connected or coordinated to maintain a spatial relationship. Such a system allows a change in the direction of the beam axis with respect to the patient without moving the patient.
"Cantilevered tabletop" means a tabletop designed such that the unsupported portion can be extended at least 100 cm beyond the support.
"Cassette holder" means a device, other than a spot-film device, that supports and/or fixes the position of an x-ray film [imaging] cassette during an x-ray exposure.
"Coefficient of variation (C)" means the ratio of the standard deviation to the mean value of a population of observations. It is estimated using the following equation:
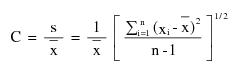
where:
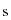 = Estimated standard deviation of the population.
= Estimated standard deviation of the population.
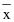 = Mean value of observations in sample;
= Mean value of observations in sample;
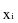 = ith observation in sample;
= ith observation in sample;
n = Number of observations sampled.
"Computed tomography (CT)" means the production of a tomogram by the acquisition and computer processing of x-ray transmission data.
"Control panel" means that part of the x-ray control upon which are mounted the switches, knobs, pushbuttons, and other hardware necessary for manually setting the technique factors.
"Cooling curve" means the graphical relationship between heat units stored and cooling time.
"Cradle" means:
(1) A removable device which supports and may restrain a patient above an x-ray table; or
(2) A device;
(i) Whose patient support structure is interposed between the patient and the image receptor during normal use;
(ii) Which is equipped with means for patient restraint; and
(iii) Which is capable of rotation about its long (longitudinal) axis.
"CT" (See "Computed tomography").
"CT gantry" means tube housing assemblies, beam-limiting devices, detectors, and the supporting structures, frames, and covers which hold and/or enclose these components.
"Cumulative air kerma" means the total air kerma accrued from the beginning of an examination or procedure and includes all contributions from fluoroscopic and radiographic irradiation.
"Detector" (See "Radiation detector")
"Diagnostic source assembly" means the tube housing assembly with a beam-limiting device attached.
"Diagnostic x-ray system" means an x-ray system designed for irradiation of any part of the human [or animal] body for the purpose of diagnosis or visualization.
"Direct scattered radiation" means that scattered radiation which has been deviated in direction only by materials irradiated by the useful beam (See "Scattered radiation").
"Dose" means the absorbed dose as defined by the International Commission on Radiation Units and Measurements. The absorbed dose, D, is the quotient of de by dm, where de is the mean energy imparted to matter of mass dm; thus D=de/dm, in units of J/kg, where the special name of the unit of absorbed dose is gray (Gy).
"Equipment" (See "X-ray equipment").
"Exposure (X)" means the quotient of dQ by dm where dQ is the absolute value of the total charge of the ions of one sign produced in air when all the electrons and positrons liberated or created by photons in air of mass dm are completely stopped in air; thus X=dQ/dm, in units of C/kg. A second meaning of exposure is the process or condition during which the x-ray tube produces x-ray radiation.
"Field emission equipment" means equipment which uses an x-ray tube in which electron emission from the cathode is due solely to the action of an electric field.
"Filter" means material placed in the useful beam to preferentially absorb selected radiations.
"Fluoroscopic air kerma display devices" means separate devices, subsystems, or components that provide the display of AKR and cumulative air kerma, respectively, required by 5.0. They include radiation detectors, if any, electronic and computer components, associated software, and data displays.
"Fluoroscopic imaging assembly" means a subsystem in which x-ray photons produce a set of fluoroscopic images or radiographic images recorded from the fluoroscopic image receptor. It includes the image receptor(s), electrical interlocks, if any, and structural material providing linkage between the image receptor and diagnostic source assembly.
"Fluoroscopic irradiation time" means the cumulative duration during an examination or procedure of operator-applied continuous pressure to the device, enabling x-ray tube activation in any fluoroscopic mode of operation.
"Fluoroscopy" means a technique for generating x-ray images and presenting them simultaneously and continuously as visible images. This term has the same meaning as the term “radioscopy” in the standards of the International Electrotechnical Commission.
"Focal spot (actual)" means the area projected on the anode of the x-ray tube bombarded by the electrons accelerated from the cathode and from which the useful beam originates.
"General purpose radiographic x-ray system" means any radiographic x-ray system which, by design, is not limited to radiographic examination of specific anatomical regions.
"Gonad shield" means a protective barrier for the testes or ovaries.
"Half-value layer (HVL)" means the thickness of specified material which attenuates the beam of radiation to an extent such that the AKR is reduced by one-half of its original value. In this definition, the contribution of all scattered radiation, other than any which might be present initially in the beam concerned, is deemed to be excluded.
"Hand-held x-ray equipment" means x-ray equipment that is designed to be hand-held during operation.
"Healing arts screening" means the testing of human beings using x-ray machines for the detection or evaluation of health indications when such tests are not specifically and individually ordered by a licensed practitioner of the healing arts legally authorized to prescribe such x-ray tests for the purpose of diagnosis or treatment.
"Heat unit" means a unit of energy equal to the product of the peak kilovoltage, milliamperes, and seconds, i.e., kVp x mA x second.
"HVL" (See "Half-value layer").
"Image intensifier" means a device, installed in its housing, which instantaneously converts an x-ray pattern into a corresponding light image of higher intensity.
"Image receptor" means any device, such as a fluorescent screen, radiographic film, x-ray image intensifier tube, solid-state detector, or gaseous detector which transforms incident x-ray photons either into a visible image or into another form which can be made into a visible image by further transformations. In those cases where means are provided to preselect a portion of the image receptor, the term “image receptor” shall mean the preselected portion of the device.
"Image receptor support device" means, for mammography x-ray systems, that part of the system designed to support the image receptor during a mammographic examination and to provide a primary protective barrier.
"Irradiation" means the exposure of matter to ionizing radiation.
"Isocenter" means the center of the smallest sphere through which the beam axis passes when the equipment moves through a full range of rotations about its common center.
"Kerma" means the quantity defined by the International Commission on Radiation Units and Measurements. The kerma, K, is the quotient of dEtr by dm, where dEtr is the sum of the initial kinetic energies of all the charged participles liberated by uncharged particles in a mass dm of material; thus K=dEtr/dm, in units of J/kg, where the special name for the unit of kerma is gray (Gy). When the material is air, the quantity is referred to as “air kerma.”
"Kilovolts peak" (See "Peak tube potential").
"kV" means kilovolts.
"kVp" (See "Peak tube potential").
"kWs" means kilowatt second.
"Last image hold (LIH) radiograph" means an image obtained either by retaining one or more fluoroscopic images, which may be temporarily integrated, at the end of a fluoroscopic exposure or by initiating a separate and distinct radiographic exposure automatically and immediately in conjunction with termination of the fluoroscopic exposure.
"Lateral fluoroscope" means the x-ray tube and image receptor combination in a biplane system dedicated to the lateral projection. It consists of the lateral x-ray tube housing assembly and the lateral image receptor that are fixed in position relative to the table with the x-ray beam axis parallel to the plane of the table.
"Lead equivalent" means the thickness of lead affording the same attenuation, under specified conditions, as the material in question.
"Leakage radiation" means radiation emanating from the diagnostic source assembly except for:
(1) The useful beam; and
(2) Radiation produced when the exposure switch or timer is not activated.
"Leakage technique factors" means the technique factors associated with the diagnostic source assembly which are used in measuring leakage radiation. They are defined as follows:
(1) For diagnostic source assemblies intended for capacitor energy storage equipment, the maximum-rated peak tube potential and the maximum-rated number of exposures in an hour for operation at the maximum-rated peak tube potential with the quantity of charge per exposure being 10 millicoulombs (or 10 mAs) or the minimum obtainable from the unit, whichever is larger;
(2) For diagnostic source assemblies intended for field emission equipment rated for pulsed operation, the maximum-rated peak tube potential and the maximum-rated number of x-ray pulses in an hour for operation at the maximum-rated peak tube potential;
(3) For all other diagnostic source assemblies, the maximum-rated peak tube potential and the maximum-rated continuous tube current for the maximum-rated peak tube potential.
"Licensed Practitioner" means an individual licensed to practice medicine, dentistry, dental hygiene, podiatry, chiropractic, or osteopathy in this State.
"Light field" means that area of the intersection of the light beam from the beam-limiting device and one of the set of planes parallel to and including the plane of the image receptor, whose perimeter is the locus of points at which the illumination is one-fourth of the maximum in the intersection.
"Line-voltage regulation" means the difference between the no-load and the load line potentials expressed as a percent of the load line potential , as follows:
Percent line-voltage regulation = 100 (Vn-Vl)/Vl
where:
Vn = No-load line potential; and
Vl = Load line potential.
"mA" means milliampere.
“mAs" means milliampere second.
"Mobile x-ray equipment" (See "X-ray equipment").
"Mode of operation" means, for fluoroscopic systems, a distinct method of fluoroscopy or radiography provided by the manufacturer and selected with a set of several technique factors or other control settings uniquely associated with the mode. The set of distinct technique factors and control settings for the mode may be selected by the operation of a single control. Examples of distinct modes of operation include normal fluoroscopy (analog or digital), high-level control fluoroscopy, cineradiography (analog and digital), digital subtraction angiography, electronic radiography using the fluoroscopic image receptor, and photospot recording. In a specific mode of operation, certain system variables affecting kerma, AKR, or image quality, such as image magnification, x-ray field size, pulse rate, pulse duration, number of pulses, source-image receptor distance (SID), or optical aperture, may be adjustable or may vary; their variation per se does not comprise a mode of operation different from the one that has been selected.
"Movable tabletop" means a tabletop which, when assembled for use, is capable of movement with respect to its supporting structure within the plane of the tabletop.
"Non-image-intensified fluoroscopy" means fluoroscopy using only a fluorescent screen.
"Patient" means an individual or animal subjected to healing arts examination, diagnosis or treatment.
"PBL" See "Positive beam limitation."
"Peak tube potential" means the maximum value of the potential difference across the x-ray tube during an exposure.
"Phantom" means a volume of material behaving in a manner similar to tissue with respect to the attenuation and scattering of radiation. This requires that both the atomic number (Z) and the density of the material be similar to that of tissue.
"PID" (See "Position indicating device").
"Portable x-ray equipment" (See "X-ray equipment").
"Position indicating device" means a device on dental x-ray equipment used to indicate the beam position and to establish a definite source-surface (skin) distance. It may or may not incorporate or serve as a beam-limiting device.
"Positive beam limitation" means the automatic or semi-automatic adjustment of an x-ray beam to the size of the selected image receptor, whereby exposures cannot be made without such adjustment.
"Primary protective barrier" means the material, excluding filters, placed in the useful beam to reduce the radiation exposure [beyond the patient and cassette holder] for protection purposes.
"Protective apron" means an apron made of radiation absorbing materials used to reduce radiation exposure.
"Protective glove" means a glove made of radiation absorbing materials used to reduce radiation exposure.
"Pulsed mode" means operation of the x-ray system such that the x-ray tube current is pulsed by the x-ray control to produce one or more exposure intervals of duration less than one-half second.
“Qualified expert” means an individual who has demonstrated to the satisfaction of the Agency that such individual possesses the knowledge and training to measure ionizing radiation, to evaluate safety techniques, and to advise regarding radiation protection needs, for example, individuals certified in the appropriate field by the American Board of Radiology, or the American Board of Health Physics, or the American Board of Medical Physics, or those having equivalent qualifications. With reference to the calibration of radiation therapy equipment, an individual, in addition to the above qualifications, must be qualified in accordance with Part X.
"Qualified medical physicist" means an individual who meets the requirements specified in Part X.
"Quick change x-ray tube" means an x-ray tube designed for use in its associated tube housing such that:
(1) The tube cannot be inserted in its housing in a manner that would result in noncompliance of the system with the requirements of 6.0;
(2) The focal spot position will not cause noncompliance with the provisions of this section or;
(3) The shielding within the tube housing cannot be displaced; and
(4) Any removal and subsequent replacement of a beam-limiting device during reloading of the tube in the tube housing will not result in noncompliance of the x-ray system with the applicable field limitation and alignment requirements of 6.0.
"Radiation detector" means a device which in the presence of radiation provides a signal or other indication suitable for use in measuring one or more quantities of incident radiation.
"Radiation therapy simulation system" means a radiographic or fluoroscopic x‑ray system intended for localizing the volume to be exposed during radiation therapy and confirming the position and size of the therapeutic irradiation field.
"Radiograph" means an image receptor on which the image is created directly or indirectly by an x-ray pattern and results in a permanent record.
"Radiography" means a technique for generating and recording an x-ray pattern for the purpose of providing the user with an image(s) after termination of the exposure.
"Rated line voltage" means the range of potentials, in volts, of the supply line specified by the manufacturer at which the x-ray machine is designed to operate.
"Rated output current" means the maximum allowable load current of the x-ray high-voltage generator.
"Rating" means the operating limits specified by the manufacturer.
"Recording" means producing a retrievable form of an image resulting from x-ray photons.
"Scan" means the complete process of collecting x-ray transmission data for the production of a tomogram. Data may be collected simultaneously during a single scan for the production of one or more tomograms.
"Scan time" means the period of time between the beginning and end of x-ray transmission data accumulation for a single scan.
"Scattered radiation" means radiation that, during passage through matter, has been deviated in direction (See "Direct scattered radiation").
"Shutter" means a device attached to the tube housing assembly which can intercept the entire cross sectional area of the useful beam and which has a lead equivalency not less than that of the tube housing assembly.
"SID" (See "Source-image receptor distance").
"Solid state x-ray imaging device" means an assembly, typically in a rectangular panel configuration, that intercepts x-ray photons and converts the photon energy into a modulated electronic signal representative of the x-ray intensity over the area of the imaging device. The electronic signal is then used to create an image for display and/or storage.
"Source" means the focal spot of the x-ray tube.
"Source-image receptor distance" means the distance from the source to the center of the input surface of the image receptor.
"Source-skin distance (SSD)" means the distance from the source to the center of the entrant x-ray field in the plane tangent to the patient skin surface.
"Spot film" means a radiograph which is made during a fluoroscopic examination to permanently record conditions which exist during that fluoroscopic procedure.
"Spot-film device" means a device intended to transport and/or position a radiographic image receptor between the x-ray source and fluoroscopic image receptor. It includes a device intended to hold a cassette over the input end of the fluoroscopic image receptor for the purpose of producing a radiograph.
"Stationary tabletop" means a tabletop which, when assembled for use, is incapable of movement with respect to its supporting structure within the plane of the tabletop.
"Stationary x-ray equipment" (See "X-ray equipment").
"Stray radiation" means the sum of leakage and scattered radiation.
"Technique factors" means the following conditions of operation:
(1) For capacitor energy storage equipment, peak tube potential in kilovolts (kV) and quantity of charge in milliampere-seconds (mAs);
(2) For field emission equipment rated for pulsed operation, peak tube potential in kV, and number of x-ray pulses;
(3) For CT equipment designed for pulsed operation, peak tube potential in kV, scan time in seconds, and either tube current in milliamperes (mA), x-ray pulse width in seconds, and the number of x-ray pulses per scan, or the product of tube current, x-ray pulse width, and the number of x-ray pulses in mAs;
(4) For CT equipment not designed for pulsed operation, peak tube potential in kV, and either tube current in mA and scan time in seconds, or the product of tube current and exposure time in mAs and the scan time when the scan time and exposure time are equivalent; and
(5) For all other equipment, peak tube potential in kV, and either tube current in mA and exposure time in seconds, or the product of tube current and exposure time in mAs.
"Tomogram" means the depiction of the x-ray attenuation properties of a section through the body.
"Tube" means an x-ray tube, unless otherwise specified.
"Tube housing assembly" means the tube housing with tube installed. It includes high-voltage and/or filament transformers and other appropriate elements when such are contained within the tube housing.
"Tube rating chart" means the set of curves which specify the rated limits of operation of the tube in terms of the technique factors.
"Useful beam" means the radiation which passes through the tube housing port and the aperture of the beam limiting device when the exposure switch or timer is activated.
"Variable-aperture beam-limiting device" means a beam-limiting device which has capacity for stepless adjustment of the x-ray field size at a given SID.
"Visible area" means that portion of the input surface of the image receptor over which incident x-ray photons are producing a visible image.
"X-ray control" means a device which controls input power to the x-ray high-voltage generator and/or the x-ray tube. It includes equipment such as timers, phototimers, automatic brightness stabilizers, and similar devices, which control the technique factors of an x-ray exposure.
"X-ray exposure control" means a device, switch, button or other similar means by which an operator initiates and/or terminates the radiation exposure. The x-ray exposure control may include such associated equipment as timers and back-up timers.
"X-ray equipment" means an x-ray system, subsystem, or component thereof. Types of x-ray equipment are as follows:
(1) "Mobile x-ray equipment" means x-ray equipment mounted on a permanent base with wheels and/or casters for moving while completely assembled.
(2) "Portable x-ray equipment" means x-ray equipment designed to be hand-carried.
(3) "Stationary x-ray equipment" means x-ray equipment which is installed in a fixed location.
"X-ray field" means that area of the intersection of the useful beam and any one of a set of planes parallel to and including the plane of the image receptor, whose perimeter is the locus of points at which the AKR is one-fourth of the maximum in the intersection.
"X-ray high-voltage generator" means a device which transforms electrical energy from the potential supplied by the x-ray control to the tube operating potential. The device may also include means for transforming alternating current to direct current, filament transformers for the x-ray tube(s), high-voltage switches, electrical protective devices, and other appropriate elements.
"X-ray subsystem" means any combination of two or more components of an x-ray system for which there are requirements specified in this section and 4.0, 5.0, 6.0.
"X-ray system" means an assemblage of components for the controlled production of x-rays. It includes minimally an x-ray high-voltage generator, an x-ray control, a tube housing assembly, a beam-limiting device, and the necessary supporting structures. Additional components which function with the system are considered integral parts of the system.
"X-ray table" means a patient support device with its patient support structure (tabletop) interposed between the patient and the image receptor during radiography and/or fluoroscopy. This includes, but is not limited to, any stretcher equipped with a radiolucent panel and any table equipped with a cassette tray (or bucky), cassette tunnel, fluoroscopic image receptor, or spot-film device beneath the tabletop.
"X-ray tube" means any electron tube which is designed for the conversion of electrical energy into x-ray energy.
3.1 Radiation Safety Requirements. The registrant, licensee, shall be responsible for directing the operation of the x-ray system(s) under his administrative control. The registrant, licensee, or the registrant's, licensee’s, agent shall assure that the requirements of these regulations are met in the operation of the x-ray system(s).
3.1.1 An x-ray system which does not meet the provisions of these regulations shall not be operated for diagnostic purposes.
3.1.2 Individuals who will be operating the x-ray systems shall meet the Agency’s qualifications to conduct the practice of radiologic technology.
3.1.3 A chart shall be provided in the vicinity of the diagnostic x-ray system's control panel which specifies, for all examinations performed with that system, the following information:
3.1.3.1 Patient's body part and anatomical size, or body part thickness, or age (for pediatrics), versus technique factors to be utilized;
3.1.3.2 Type and size of the image receptor to be used;
3.1.3.3 Type and size of the image receptor combination to be used, if any;
3.1.3.4 Source to image receptor distance to be used (except for dental intraoral radiography);
3.1.3.5 Type and location of placement of patient shielding (e.g., gonad, etc.) to be used; and
3.1.3.6 For mammography, indication of kVp/target/filter combination.
3.1.4 The registrant [licensee] of a facility shall create and make available to x-ray operators written safety procedures, including patient holding and any restrictions of the operating technique required for the safe operation of the particular x-ray system. The operator shall be able to demonstrate familiarity with these procedures.
3.1.5 Except for patients who cannot be moved out of the room, only the staff, ancillary personnel or other persons required for the medical procedure or training shall be in the room during the radiographic exposure. Other than the patient being examined:
3.1.5.1 All individuals shall be positioned such that no part of the body will be struck by the useful beam unless protected by not less than 0.5 millimeter lead equivalent material;
3.1.5.2 The x-ray operator, other staff, ancillary personnel, and other persons required for the medical procedure shall be protected from the direct scatter radiation by protective aprons or whole body protective barriers of not less than 0.25 millimeter lead equivalent material;
3.1.5.3 Human patients who cannot be removed from the room shall be protected from the direct scatter radiation by whole body protective barriers of not less than 0.25 millimeter lead equivalent material or shall be so positioned that the nearest portion of the body is at least 2 meters from both the tube head and the nearest edge of the image receptor.
3.1.6 Gonad shielding of not less than 0.5 millimeter lead equivalent material shall be used for human patients, who have not passed the reproductive age, during radiographic procedures in which the gonads are in the useful beam, except for cases in which this would interfere with the diagnostic procedure.
3.1.7 Individuals shall not be exposed to the useful beam except for healing arts purposes and unless such exposure has been authorized by a licensed practitioner of the healing arts. This provision specifically prohibits deliberate exposure for the following purposes:
3.1.7.1 Exposure of an individual for training, demonstration, or other non-healing arts purposes; and
3.1.7.2 Exposure of an individual for the purpose of healing arts screening except as authorized by 3.1.11.
3.1.8 When a patient or image receptor must be provided with auxiliary support during a radiation exposure:
3.1.8.1 Mechanical holding devices shall be used when the technique permits. The written safety procedures, required by 3.1.4, shall list individual projections where holding devices cannot be utilized;
3.1.8.2 Written safety procedures, as required by 3.1.4, shall indicate the requirements for selecting a holder and the procedure the holder shall follow;
3.1.8.3 The human holder shall be instructed in personal radiation safety and protected as required by 3.1.5.;
3.1.8.4 No individual shall be used routinely to hold image receptor or patients;
3.1.8.5 In those cases where the patient must hold the image receptor, except during intraoral examinations, any portion of the body other than the area of clinical interest struck by the useful beam shall be protected by not less than 0.5 millimeter lead equivalent material; and
3.1.8.6 Each facility shall have leaded aprons and gloves available in sufficient numbers to provide protection for all personnel who are involved with x-ray operations and who are otherwise not shielded.
3.1.9 Procedures and auxiliary equipment designed to minimize patient and personnel exposure commensurate with the needed diagnostic information shall be utilized.
3.1.9.1 The fastest imaging system consistent with the diagnostic objective of the examinations shall be used. Film cassettes without intensifying screens shall not be used for any routine diagnostic radiological imaging, with the exception of veterinary radiography and standard film packets for intraoral use in dental radiography.
3.1.9.2 The radiation exposure to the patient shall be the minimum exposure required to produce images of good diagnostic quality.
3.1.9.3 Portable or mobile x-ray equipment shall be used only for examinations where it is impractical to transfer the patient(s) to a stationary x-ray installation. The use of hand held devices must be approved by the Agency utilizing the criteria in Appendix B.
3.1.9.4 X-ray systems subject to 6.0 shall not be utilized in procedures where the source to patient distance is less than 30 centimeters, except for veterinary systems.
3.1.9.5 If grids are used between the patient and the image receptor to decrease scatter to the film and improve contrast, the grid shall:
3.1.9.5.1 Be positioned properly, i.e., tube side facing the right direction, and grid centered to the central ray;
3.1.9.5.2 If the grid is of the focused type, be of the proper focal distance for the SIDs being used.
3.1.10 All individuals who are associated with the operation of an x-ray system are subject to the requirements of D.1201, D.1207 and D.1208 of these regulations.
3.1.11 Healing Arts Screening. Any person proposing to conduct a healing arts screening program shall not initiate such a program without prior approval of the Agency. When requesting such approval, that person shall submit the information outlined in Appendix A of this Part. If any information submitted to the Agency becomes invalid or outdated, the Agency shall be immediately notified.
3.1.12 Information and Maintenance Record and Associated Information. The registrant [licensee] shall maintain the following information for each x-ray system for inspection by the Agency:
3.1.12.1 Model and serial numbers of all major components, and user's manuals for those components;
3.1.12.2 Tube rating charts and cooling curves;
3.1.12.3 Records of surveys, calibrations, maintenance, and modifications performed on the x-ray system(s); and
3.1.12.4 A copy of all correspondence with this Agency regarding that x-ray system.
3.1.13 X-Ray Utilization Record. Except for veterinary facilities, each facility shall maintain a record containing the patient's name, the type of examinations, and the dates the examinations were performed. When the patient or film must be provided with human auxiliary support, the name of the human holder should be recorded.
3.2 X-Ray Film Processing Facilities and Practices.
3.2.1 Each installation using a radiographic x-ray system and using analog image receptors (e.g. radiographic film) shall have available suitable equipment for handling and processing radiographic film in accordance with the following provisions:
3.2.1.1 Manually developed film:
3.2.1.2 Processing tanks shall be constructed of mechanically rigid, corrosion resistant material; and
3.2.1.3 The temperature of solutions in the tanks shall be maintained within the range of 60o F to 80o F (16o C to 27o C). Film shall be developed in accordance with the time-temperature relationships recommended by the film manufacturer, or, in the absence of such recommendations, with the following time-temperature chart:
Time-Temperature Chart | ||
Thermometer Reading (Degrees) | Minimum Developing Time (Minutes) | |
oC | oF | |
26.7 | 80 | 2 |
26.1 | 79 | 2 |
25.6 | 78 | 2½ |
25.0 | 77 | 2½ |
24.4 | 76 | 3 |
23.9 | 75 | 3 |
23.3 | 74 | 3½ |
22.8 | 73 | 3½ |
22.2 | 72 | 4 |
21.7 | 71 | 4 |
21.1 | 70 | 4½ |
20.6 | 69 | 4½ |
20.0 | 68 | 5 |
19.4 | 67 | 5½ |
18.9 | 66 | 5½ |
18.3 | 65 | 6 |
17.8 | 64 | 6½ |
17.2 | 63 | 7 |
16.7 | 62 | 8 |
16.1 | 61 | 8½ |
15.6 | 60 | ½ |
3.2.1.4 Devices shall be utilized which will indicate the actual temperature of the developer and signal the passage of a preset time appropriate to the developing time required.
3.2.1.5 Automatic processors and other closed processing systems: Films shall be developed in accordance with the time-temperature relationships recommended by the film manufacturer; in the absence of such recommendations, the film shall be developed using the following chart:
Developer Temperature | Minimum Immersion Timea/ | |
oC | oF | Seconds |
35.5 | 96 | 19 |
35 | 95 | 20 |
34.5 | 94 | 21 |
34 | 93 | 22 |
33.5 | 92 | 23 |
33 | 91 | 24 |
32 | 90 | 25 |
31.5 | 89 | 26 |
31 | 88 | 27 |
30.5 | 87 | 28 |
30 | 86 | 29 |
29.5 | 85 | 30 |
a/ Immersion time only, no crossover time included. | ||
3.2.1.6 Processing deviations from the requirements of 3.2.1 shall be documented by the registrant [licensee] in such manner that the requirements are shown to be met or exceeded (e.g., extended processing, and special rapid chemistry).
3.3 Other Requirements.
3.3.1 Pass boxes, if provided, shall be so constructed as to exclude light from the darkroom when cassettes are placed in or removed from the boxes, and shall incorporate adequate shielding from stray radiation to prevent exposure of undeveloped film.
3.3.2 The darkroom shall be light tight and use proper safelighting such that any film type in use exposed in a cassette to x-radiation sufficient to produce an optical density from 1 to 2 when processed shall not suffer an increase in density greater than 0.1 (0.05 for mammography) when exposed in the darkroom for 2 minutes with all safelights on. If used, daylight film handling boxes shall preclude fogging of the film.
3.3.3 Darkrooms typically used by more than one individual shall be provided a method to prevent accidental entry while undeveloped films are being handled or processed.
3.3.4 Film shall be stored in a cool, dry place and shall be protected from exposure to stray radiation. Film in open packages shall be stored in a light tight container.
3.3.5 Film cassettes and intensifying screens shall be inspected periodically and shall be cleaned and replaced as necessary to best assure radiographs of good diagnostic quality.
3.3.6 Outdated x-ray film shall not be used for diagnostic radiographs, unless the film has been stored in accordance with the manufacturer's recommendations and a sample of the film passes a sensitometric test for normal ranges of base plus fog and speed.
3.3.7 Film developing solutions shall be prepared in accordance with the directions given by the manufacturer, and shall be maintained in strength by replenishment or renewal so that full development is accomplished within the time specified by the manufacturer.
In addition to other requirements of this Part, all diagnostic x‑ray systems shall meet the following requirements:
4.1 Warning Label. The control panel containing the main power switch shall bear the warning statement, legible and accessible to view: "WARNING: This x-ray unit may be dangerous to patient and operator unless safe exposure factors, operating instructions and maintenance schedules are observed."
4.2 Leakage Radiation from the Diagnostic Source Assembly. The leakage radiation from the diagnostic source assembly measured at a distance of 1 meter in any direction from the source shall not exceed 0.88 milligray (mGy) air kerma (vice 100 milliroentgen (mR) exposure) in 1 hour when the x-ray tube is operated at its leakage technique factors. If the maximum rated peak tube potential of the tube housing assembly is greater than the maximum rated peak tube potential for the diagnostic source assembly, positive means shall be provided to limit the maximum x-ray tube potential to that of the diagnostic source assembly. Compliance shall be determined by measurements averaged over an area of 100 square centimeters with no linear dimension greater than 20 centimeters.
4.3 Radiation from Components Other Than the Diagnostic Source Assembly. The radiation emitted by a component other than the diagnostic source assembly shall not exceed an air kerma of 18 microgray (vice 2 milliroentgens exposure) in 1 hour at 5 centimeters from any accessible surface of the component when it is operated in an assembled x-ray system under any conditions for which it was designed. Compliance shall be determined by measurements averaged over an area of 100 square centimeters with no linear dimension greater than 20 centimeters.
4.4 Beam Quality.
4.4.1 Half-Value Layer (HVL).
4.4.1.1 The HVL of the useful beam for a given x-ray tube potential shall not be less than the values shown in Table 1 in this section under the heading “Specified Dental Systems,” for any dental x-ray system designed for use with intraoral image receptors and manufactured after December 1, 1980; under the heading, “I-Other X-Ray Systems,” for any dental x-ray system designed for use with intraoral image receptors and manufactured before or on December 1, 1980, and all other x-ray systems subject to this section and manufactured before June 10, 2006; and under the heading, “II-Other X-Ray Systems,” for all x-ray systems, except dental x-ray systems designed for use with intraoral image receptors, subject to this section and manufactured on or after June 10, 2006. If it is necessary to determine such half-value layer at an x-ray tube potential which is not listed in Table 1 of this section, linear interpolation or extrapolation may be made. Positive means shall be provided to ensure that at least the minimum filtration needed to achieve beam quality requirements is in the useful beam during each exposure. In the case of a system, which is to be operated with more than one thickness of filtration, this requirement can be met by a filter interlocked with the kilovoltage selector which will prevent x-ray emissions if the minimum required filtration is not in place.
X-Ray Tube Voltage (kilovolt peak) | ||||
Design Operating Range | Measured Operating Potential | Minimum HVL (mm in Aluminum) | ||
Specified Dental Systems \1\ | Other X-Ray Systems\2\ | Other X-Ray Systems\3\ | ||
Below 51 | 30 | 1.5 | 0.3 | 0.3 |
40 | 1.5 | 0.4 | 0.4 | |
50 | 1.5 | 0.5 | 0.5 | |
51 to 70 | 51 | 1.5 | 1.2 | 1.3 |
60 | 1.5 | 1.3 | 1.5 | |
70 | 1.5 | 1.5 | 1.8 | |
Above 70 | 71 | 2.1 | 2.1 | 2.5 |
80 | 2.3 | 2.3 | 2.9 | |
90 | 2.5 | 2.5 | 3.2 | |
100 | 2.7 | 2.7 | 3.6 | |
110 | 3.0 | 3.0 | 3.9 | |
120 | 3.2 | 3.2 | 4.3 | |
130 | 3.5 | 3.5 | 4.7 | |
140 | 3.8 | 3.8 | 5.0 | |
150 | 4.1 | 4.1 | 5.4 | |
\1\ Dental x-ray systems designed for use with intraoral image receptors and manufactured after December 1, 1980. \2\ Dental x-ray systems designed for use with intraoral image receptors and manufactured before or on December 1, 1980, and all other x-ray systems subject to this section and manufactured before June 10, 2006. \3\ All x-ray systems, except dental x-ray systems designed for use with intraoral image receptors, subject to this section and manufactured on or after June 10, 2006. | ||||
4.4.1.2 Optional filtration. Fluoroscopic systems manufactured on or after June 10, 2006, incorporating an x-ray tube(s) with a continuous output of 1 kilowatt or more and an anode heat storage capacity of 1 million heat units or more shall provide the option of adding x-ray filtration to the diagnostic source assembly in addition to the amount needed to meet the half-value layer provisions of this subsection. The selection of this additional x-ray filtration shall be either at the option of the user or automatic as part of the selected mode of operation. A means of indicating which combination of additional filtration is in the x-ray beam shall be provided.
4.4.1.3 Measuring compliance. For capacitor energy storage equipment, compliance shall be determined with the maximum selectable quantity of charge per exposure.
4.4.1.4 Aluminum equivalent of material between patient and image receptor. Except when used in a CT x-ray system, the aluminum equivalent of each of the items listed in Table 2 in this paragraph, which are used between the patient and the image receptor, may not exceed the indicated limits. Compliance shall be determined by x-ray measurements made at a potential of 100 kilovolts peak and with an x-ray beam that has an HVL specified in Table 1 of this section for the potential. This requirement applies to front panel(s) of cassette holders and film changers provided by the manufacturer for patient support or for prevention of foreign object intrusions. It does not apply to screens and their associated mechanical support panels or grids. Table 2 follows:
Item | Maximum Aluminum Equivalent (millimeters) |
Front panel(s) of cassette holders (total of all) Film panel(s) of film changer (total of all) Cradle Tabletop, stationary, without articulated joints Tabletop, movable, without articulated joint(s) (including stationary subtop) Tabletop, with radiolucent panel having one articulated joint Tabletop, with radiolucent panel having two or more articulated joints Tabletop, cantilevered Tabletop, radiation therapy simulator | 1.2 1.2 2.3 1.2 1.7 1.7 2.3 2.3 5.0 |
4.5 Battery charge indicator. On battery-powered generators, visual means shall be provided on the control panel to indicate whether the battery is in a state of charge adequate for proper operation.
4.6 Modification of certified diagnostic x-ray components and systems.
4.7 Diagnostic x-ray components and systems certified in accordance with 21 CFR Part 1020 shall not be modified such that the component or system fails to comply with any applicable provision of this Part.
4.8 The owner of a diagnostic x-ray system who uses the system in a professional or commercial capacity may modify the system provided the modification does not result in the failure of the system or component to comply with the applicable requirements of this Part. The owner who causes such modification need not submit the reports required by this Part, provided the owner records the date and the details of the modification in the system records and maintains this information, and provided the modification of the x-ray system does not result in a failure to comply with this Part. Such system modifications should be per suggested manufacturer’s specifications.
4.9 Multiple Tubes. Where two or more radiographic tubes are controlled by one exposure switch, the tube or tubes which have been selected shall be clearly indicated prior to initiation of the exposure. This indication shall be both on the x-ray control panel and at or near the tube housing assembly which has been selected.
4.10 Mechanical Support of Tube Head. The tube housing assembly supports shall be adjusted such that the tube housing assembly will remain stable during an exposure unless tube housing movement is a designed function of the x-ray system.
4.11 Technique Indicators.
4.11.1 For x-ray equipment capable of displaying technique factors, the technique factors to be used during an exposure shall be indicated before the exposure begins. If automatic exposure controls are used, the technique factors which are set prior to the exposure shall be indicated.
4.11.2 The requirement of 4.11.1 may be met by permanent markings on equipment having fixed technique factors. Indication of technique factors shall be visible from the operator's position except in the case of spot films made by the fluoroscopist.
4.12 Maintaining Compliance. Diagnostic x-ray systems and their associated components used on humans and certified pursuant to the Federal X-Ray Equipment Performance Standard (21 CFR Part 1020) shall be maintained in compliance with applicable requirements of that standard.
4.13 Locks. All position locking, holding, and centering devices on x-ray system components and systems shall function as intended.
The provisions of this Part apply to equipment for fluoroscopic imaging or for recording images from the fluoroscopic image receptor, except computed tomography x-ray systems manufactured on or after November 29, 1984.
5.1 Primary Protective Barrier.
5.1.1 Limitation of useful beam. The fluoroscopic imaging assembly shall be provided with a primary protective barrier which intercepts the entire cross section of the useful beam at any SID. The x-ray tube used for fluoroscopy shall not produce x-rays unless the barrier is in position to intercept the entire useful beam. The AKR due to transmission through the barrier with the attenuation block in the useful beam combined with radiation from the fluoroscopic imaging receptor shall not exceed 3.34x10-3 percent of the entrance AKR, at a distance of 10 cm from any accessible surface of the fluoroscopic imaging assembly beyond the plane of the image receptor. Radiation therapy simulation systems shall be exempt from this requirement provided the systems are intended only for remote control operation.
5.1.2 Measuring compliance. The AKR shall be measured in accordance with 5.3.2. The AKR due to transmission through the primary barrier combined with radiation from the fluoroscopic image receptor shall be determined by measurements averaged over an area of 100 square cm with no linear dimension greater than 20 cm. If the source is below the tabletop, the measurement shall be made with the input surface of the fluoroscopic imaging assembly positioned 30 cm above the tabletop. If the source is above the tabletop and the SID is variable, the measurement shall be made with the end of the beam-limiting device or spacer as close to the tabletop as it can be placed, provided that it shall not be closer than 30 cm. Movable grids and compression devices shall be removed from the useful beam during the measurement. For all measurements, the attenuation block shall be positioned in the useful beam 10 cm from the point of measurement of entrance AKR and between this point and the input surface of the fluoroscopic imaging assembly.
5.2 Field Limitation.
5.2.1 Angulation. For fluoroscopic equipment manufactured after February 25, 1978, when the angle between the image receptor and the beam axis of the x-ray beam is variable, means shall be provided to indicate when the axis of the x-ray beam is perpendicular to the plane of the image receptor. Compliance with 5.2.4 and 5.2.5 shall be determined with the beam axis indicated to be perpendicular to the plane of the image receptor.
5.2.2 Further means for limitation. Means shall be provided to permit further limitation of the x-ray field to sizes smaller than the limits of 5.2.4 and 5.2.5. Beam-limiting devices manufactured after May 22, 1979, and incorporated in equipment with a variable SID and/or capability of a visible area of greater than 300 square cm, shall be provided with means for stepless adjustment of the x-ray field. Equipment with a fixed SID and the capability of a visible area of no greater than 300 square cm shall be provided with either stepless adjustment of the x-ray field or with a means to further limit the x-ray field size at the plane of the image receptor to 125 square cm or less. Stepless adjustment shall, at the greatest SID, provide continuous field sizes from the maximum obtainable to a field size containable in a square of 5 cm by 5 cm. This paragraph does not apply to non-image-intensified fluoroscopy.
5.2.3 Non-image-intensified fluoroscopy. The x-ray field produced by non-image-intensified fluoroscopic equipment shall not extend beyond the entire visible area of the image receptor. Means shall be provided for stepless adjustment of field size. The minimum field size, at the greatest SID, shall be containable in a square of 5 cm by 5 cm.
5.2.4 Fluoroscopy and radiography using the fluoroscopic imaging assembly with inherently circular image receptors.
5.2.4.1 For fluoroscopic equipment manufactured before June 10, 2006, other than radiation therapy simulation systems, the following applies:
5.2.4.1.1 Neither the length nor width of the x-ray field in the plane of the image receptor shall exceed that of the visible area of the image receptor by more than 3 percent of the SID. The sum of the excess length and the excess width shall be no greater than 4 percent of the SID.
5.2.4.1.2 For rectangular x-ray fields used with circular image receptors, the error in alignment shall be determined along the length and width dimensions of the x-ray field which pass through the center of the visible area of the image receptor.
5.2.4.2 For fluoroscopic equipment manufactured on or after June 10, 2006, other than radiation simulation systems, the maximum area of the x-ray field in the plane of the image receptor shall conform with one of the following requirements:
5.2.4.2.1 When any linear dimension of the visible area of the image receptor measured through the center of the visible area is less than or equal to 34 cm in any direction, at least 80 percent of the area of the x-ray field overlaps the visible area of the image receptor, or
5.2.4.2.2 When any linear dimension of the visible area of the image receptor measured through the center of the visible area is greater than 34 cm in any direction, the x-ray field measured along the direction of greatest misalignment with the visible area of the image receptor does not extend beyond the edge of the visible area of the image receptor by more than 2 cm.
5.2.5 Fluoroscopy and radiography using fluoroscopic imaging assembly with inherently rectangular image receptors. For x-ray systems manufactured on or after June 10, 2006, the following applies:
5.2.5.1 Neither the length nor width of the x-ray field in the plane of the image receptor shall exceed that of the visible area of the image receptor by more than 3 percent of the SID. The sum of the excess length and the excess width shall be no greater than 4 percent of the SID.
5.2.5.2 The error in alignment shall be determined along the length and width dimensions of the x-ray field which pass through the center of the visible area of the image receptor.
5.3 If the fluoroscopic x-ray field size is adjusted automatically as the SID or image receptor size is changed, a capability may be provided for overriding the automatic adjustment in case of system failure. If it is so provided, a signal visible at the fluoroscopist’s position shall indicate whenever the automatic field adjustment is overridden. Each such system failure override switch shall be clearly labeled as follows:
FOR X-RAY FIELD LIMITATION SYSTEM FAILURE
5.3.1 Activation of Tube. X-ray production in the fluoroscopic mode shall be controlled by a device which requires continuous pressure by the operator for the entire time of any exposure. When recording serial radiographic images from the fluoroscopic image receptor, the operator shall be able to terminate the x-ray exposure(s) at any time, but means may be provided to permit completion of any single exposure of the series in process.
5.3.2 Air Kerma Rates. For fluoroscopic equipment, the following requirements apply:
5.4 Fluoroscopic equipment manufactured before May 19, 1995.
5.4.1 Equipment provided with automatic exposure rate control (AERC) shall not be operable at any combination of tube potential and current that will result in an AKR in excess of 88 mGy per minute (vice 10 R/min exposure rate) at the measurement point specified in 5.7, except as specified in 5.5.1.
5.4.2 Equipment provided without AERC shall not be operable at any combination of tube potential and current that will result in an AKR in excess of 44 mGy per minute (vice 5 R/min exposure rate) at the measurement point specified in 5.7, except as specified in 5.5.1.
5.4.3 Equipment provided with both an AERC mode and a manual mode shall not be operable at any combination of tube potential and current that will result in an AKR in excess of 88 mGy per minute (vice 10 R/min exposure rate) in either mode at the measurement point specified in 5.7, except as specified in 5.5.1.
5.4.4 Equipment may be modified in accordance with this Part to comply with 5.6. When the equipment is modified, it shall bear a label indicating the date of the modification and the statement:
5.4.4.1 Exceptions:
5.4.4.1.1 During recording of fluoroscopic images, or
5.4.4.1.2 When a mode of operation has an optional high-level control, in which case that mode shall not be operable at any combination of tube potential and current that will result in an AKR in excess of the rates specified in paragraphs (1), (2) and (3) at the measurement point specified in 5.7, unless the high-level control is activated. Special means of activation of high-level controls shall be required. The high-level control shall be operable only when continuous manual activation is provided by the operator. A continuous signal audible to the fluoroscopist shall indicate that the high-level control is being employed.
5.5 Fluoroscopic equipment manufactured on or after May 19, 1995.
5.5.1 Shall be equipped with AERC if operable at any combination of tube potential and current that results in an AKR greater than 44 mGy per minute (vice 5 R/min exposure rate) at the measurement point specified in 5.7. Provision for manual selection of technique factors may be provided.
5.5.2 Shall not be operable at any combination of tube potential and current that will result in an AKR in excess of 88 mGy per minute (vice 10 R/min exposure rate) at the measurement point specified in 5.7, except as specified in 5.6.3.
5.5.3 Exceptions:
5.5.3.1 For equipment manufactured prior to June 10, 2006, during the recording of images from a fluoroscopic image receptor using photographic film or a video camera when the x-ray source is operated in a pulsed mode.
5.5.3.2 For equipment manufactured on or after June 10, 2006, during the recording of images from the fluoroscopic image receptor for the purpose of providing the user with a recorded image(s) after termination of the exposure. Such recording does not include images resulting from a last-image-hold feature that are not recorded.
5.5.3.3 When a mode of operation has an optional high-level control and the control is activated, in which case the equipment shall not be operable at any combination of tube potential and current that will result in an AKR in excess of 176 mGy per minute (vice 20 R/min exposure rate) at the measurement point specified in 5.7. Special means of activation of high-level controls shall be required. The high-level control shall be operable only when continuous manual activation is provided by the operator. A continuous signal audible to the fluoroscopist shall indicate that the high-level control is employed.
5.6 Measuring compliance. Compliance with this subsection shall be determined as follows:
5.6.1 If the source is below the x-ray table, the AKR shall be measured at 1 cm above the tabletop or cradle.
5.6.2 If the source is above the x-ray table, the AKR shall be measured at 30 cm above the tabletop with the end of the beam-limiting device or spacer positioned as closely as possible to the point of measurement.
5.6.3 In a C-arm type of fluoroscope, the AKR shall be measured at 30 cm from the input surface of the fluoroscopic imaging assembly, with the source positioned at any available SID, provided that the end of the beam-limiting device or spacer is no closer than 30 cm from the input surface of the fluoroscopic imaging assembly.
5.6.4 In a C-arm type of fluoroscope having an SID less than 45 cm, the AKR shall be measured at the minimum SSD.
5.6.5 In a lateral type of fluoroscope, the air kerma rate shall be measured at a point 15 cm from the centerline of the x-ray table and in the direction of the x-ray source with the end of the beam-limiting device or spacer positioned as closely as possible to the point of measurement. If the tabletop is movable, it shall be positioned as closely as possible to the lateral x-ray source, with the end of the beam-limiting device or spacer no closer than 15 cm to the centerline of the x-ray table.
5.7 Exemptions. Fluoroscopic radiation therapy simulation systems are exempt from the requirements set forth in this subsection when used for therapy simulation purposes.
5.8 Reserved.
5.9 Indication of potential and current. During fluoroscopy and cinefluorography, x-ray tube potential and current shall be continuously indicated. Deviation of x-ray tube potential and current from the indicated value shall not exceed the maximum deviation as stated by the manufacturer.
5.10 Source-skin distance.
5.10.1 Means shall be provided to limit the source-skin distance to not less than 38 cm on stationary fluoroscopes and to not less than 30 cm on mobile and portable fluoroscopes. In addition, for fluoroscopes intended for specific surgical application that would be prohibited at the source-skin distances specified in this paragraph, provisions may be made for operating at shorter source-skin distances but in no case less than 20 cm.
5.10.2 For stationary, mobile, or portable C-arm fluoroscopic systems manufactured on or after June 10, 2006, having a maximum source-image receptor distance of less than 45 cm, means shall be provided to limit the source-skin distance to not less than 19 cm. Such systems shall be labeled for extremity use only. In addition, for those systems intended for specific surgical application that would be prohibited at the source-skin distance specified in this paragraph, provisions may be made for operation at shorter source-skin distances but in no case less than 10 cm.
5.11 Fluoroscopic irradiation time, display, and signal.
5.11.1 Fluoroscopic equipment manufactured before June 10, 2006:
5.11.1.1 Shall be provided with means to preset the cumulative irradiation time of the fluoroscopic tube. The maximum cumulative time of the timing device shall not exceed 5 minutes without resetting. A signal audible to the fluoroscopist shall indicate the completion of any preset cumulative irradiation time. Such signal shall continue to sound while x-rays are produced until the timing device is reset. Fluoroscopic equipment may be modified in accordance with 21 CFR 1020.30(q) to comply with the requirements of this paragraph. When the equipment is modified, it shall bear a label indicating the statement:
5.12 Modified to comply with 21 CFR 1020.32(h)(2)
5.12.1 As an alternative to the requirements of this paragraph, radiation therapy simulation systems may be provided with a means to indicate the total cumulative exposure time during which x-rays were produced, and which is capable of being reset between x-ray examinations.
5.13 For x-ray controls manufactured on or after June 10, 2006, there shall be provided for each fluoroscopic tube:
5.13.1 A display of the fluoroscopic irradiation time at the fluoroscopist’s working position. This display shall function independently of the audible signal described in this subsection. The following requirements apply:
5.13.1.1 When the x-ray tube is activated, the fluoroscopic irradiation time in minutes and tenths of minutes shall be continuously displayed and updated at least once every 6 seconds.
5.13.1.2 The fluoroscopic irradiation time shall also be displayed within 6 seconds of termination of an exposure and remain displayed until reset.
5.13.1.3 Means shall be provided to reset the display to zero prior to the beginning of a new examination or procedure.
5.13.2 A signal audible to the fluoroscopist shall sound for each passage of 5 minutes of fluoroscopic irradiation time during an examination or procedure. The signal shall sound until manually reset or, if automatically reset, for at least 2 seconds.
5.14 Mobile and portable fluoroscopes. In addition to the other requirements of this subsection, mobile and portable fluoroscopes shall provide an image receptor incorporating more than a simple fluorescent screen.
5.15 Display of last-image-hold (LIH). Fluoroscopic equipment manufactured on or after June 10, 2006, shall be equipped with means to display LIH image following termination of the fluoroscopic exposure.
5.16 For an LIH image obtained by retaining pretermination fluoroscopic images, if the number of images and method of combining images are selectable by the user, the selection shall be indicated prior to initiation of the fluoroscopic exposure.
5.17 For an LIH image obtained by initiating a separate radiographic-like exposure at the termination of fluoroscopic imaging, the technique factors for the LIH image shall be selectable prior to the fluoroscopic exposure, and the combination selected shall be indicated prior to initiation of the fluoroscopic exposure.
5.18 Means shall be provided to clearly indicate to the user whether a displayed image is the LIH radiograph or fluoroscopy. Display of the LIH radiograph shall be replaced by the fluoroscopic image concurrently with re-initiation of fluoroscopic exposure, unless separate displays are provided for the LIH radiograph and fluoroscopic images.
5.19 Displays of values of AKR and cumulative air kerma. Fluoroscopic equipment manufactured on or after June 10, 2006, shall display at the fluoroscopist’s working position the AKR and cumulative air kerma. The following requirements apply for each x-ray tube used during an examination or procedure:
5.20 When the x-ray tube is activated and the number of images produced per unit time is greater than six images per second, the AKR in mGy/min shall be continuously displayed and updated at least once every second.
5.21 The cumulative air kerma in units of mGy shall be displayed either within 5 seconds of termination of an exposure or displayed continuously and updated at least once every 5 seconds.
5.22 The display of the AKR shall be clearly distinguishable from the display of the cumulative air kerma.
5.23 The AKR and cumulative air kerma shall represent the value for conditions of free-in-air irradiation at one of the following reference locations specified according to the type of fluoroscope.
5.23.1 For fluoroscopes with x-ray source below the x-ray table, x-ray source above the table, or of lateral type, the reference location shall be the respective locations specified in 5.7.1, 5.7.2 or 5.7.5.
5.23.2 For C-arm fluoroscopes, the reference location shall be 15 cm from the isocenter toward the x-ray source along the beam axis. Alternatively, the reference location shall be at a point specified by the manufacturer to represent the location of the intersection of the x-ray beam with the patient’s skin.
5.24 Means shall be provided to reset to zero the display of cumulative air kerma prior to the commencement of a new examination or procedure.
5.25 The displayed AKR and cumulative air kerma shall not deviate from the actual values by more than ±35 percent over the range of 6 mGy/min and 100 mGy to the maximum indication of AKR and cumulative air kerma, respectively. Compliance shall be determined with an irradiation time greater than 3 seconds.
5.26 Control of Scattered Radiation.
5.26.1 Fluoroscopic table designs when combined with procedures utilized shall be such that no unprotected part of any staff or ancillary individual's body shall be exposed to unattenuated scattered radiation which originates from under the table. The attenuation required shall be not less than 0.25 millimeter lead equivalent.
5.26.2 Equipment configuration when combined with procedures shall be such that no portion of any staff or ancillary individual's body, except the extremities, shall be exposed to the unattenuated scattered radiation emanating from above the tabletop unless that individual:
5.26.2.1 Is at least 120 centimeters from the center of the useful beam; or
5.26.2.2 The radiation has passed through not less than 0.25 millimeter lead equivalent material including, but not limited to, drapes, Bucky-slot cover panel, or self-supporting curtains, in addition to any lead equivalency provided by the protective apron referred to in 3.1.5.2.
5.27 The Agency may grant exemptions to 5.24.2. where a sterile field will not permit the use of the normal protective barriers. Where the use of prefitted sterilized covers for the barriers is practical, the Agency shall not permit such exemption. See Appendix C for a suggested list of fluoroscopic procedures where such exemptions will be automatically granted.
5.28 Operator Qualifications.
5.28.1 The facility shall ensure that only a licensed practitioner of the healing arts or a radiologic technologist be allowed to operate fluoroscopic x-ray systems. A licensed practitioner includes any health practitioner of the healing Arts who is licensed in the state to diagnose and treat individuals and who operates within the scope defined in the state law. This includes physicians and physician assistants and excludes nurse practitioners.
5.28.2 All persons operating fluoroscopic x-ray systems shall have completed at least the following training before using fluoroscopy independently: 5.26.1.
5.28.2.1 Biological effects of x-ray;
5.28.2.2 Principles of radiation protection;
5.28.2.3 Factors affecting fluoroscopic outputs;
5.28.2.4 Dose reduction techniques for fluoroscopic x-ray systems;
5.28.2.5 Principles and operation of the specific fluoroscopic x-ray system(s) to be used;
5.28.2.6 Fluoroscopic and fluorographic outputs of each mode of operation on the system(s) to be used clinically; and
5.28.2.7 Applicable requirements of these regulations.
5.29 The facility shall either provide annual in-service training for all operators of fluoroscopic x-ray systems used for high dose, high risk procedures, or require evidence of annual continuing medical education in fluoroscopic radiation safety and patient dose management.
5.30 Documentation pertaining to the requirements of 5.26.2 and 5.27, shall be maintained for review for three years.
5.31 Equipment Operation.
5.31.1 All imaging formed by the use of fluoroscopic x-ray systems shall be viewed, directly or indirectly, and interpreted by a licensed practitioner of the healing arts.
5.31.2 The operation of fluoroscopic x-ray systems by radiologic technologists [or equivalent] shall be performed under the direct supervision of a licensed practitioner of the healing arts who meets the requirements of 5.26.
5.31.3 Radiologic technology students shall not be allowed to operate fluoroscopic x-ray systems unless directly supervised by a licensed practitioner of the healing arts or radiologic technologist as specified in 5.26.1.
5.31.4 Overhead fluoroscopy shall not be used as a positioning tool for general purpose radiographic examinations.
5.31.5 Facilities shall maintain a record of the cumulative fluoroscopic exposure time used and the number of fluorographic images recorded for each examination. This record shall include patient identification, type and date of examination, the fluoroscopic system used, and operator's name.
6.1 Control and indication of technique factors.
6.1.1 Visual indication. The technique factors to be used during an exposure shall be indicated before the exposure begins, except when automatic exposure controls are used, in which case the technique factors which are set prior to the exposure shall be indicated. On equipment having fixed technique factors, this requirement may be met by permanent markings. Indication of technique factors shall be visible from the operator’s position except in the case of spot films made by the fluoroscopist.
6.1.2 Timers. Means shall be provided to terminate the exposure at a preset time interval, a preset product of current and time, a preset number of pulses, or a preset radiation exposure to the image receptor.
6.1.2.1 Except during serial radiography, the operator shall be able to terminate the exposure at any time during an exposure of greater than one-half second. Except during panoramic dental radiography, termination of exposure shall cause automatic resetting of the timer to its initial setting or to zero. It shall not be possible to make an exposure when the timer is set to a zero or off position if either position is provided.
6.1.2.2 During serial radiography, the operator shall be able to terminate the x-ray exposure(s) at any time, but means may be provided to permit completion of any single exposure of the series in process.
6.2 Automatic exposure controls. When an automatic exposure control is provided:
6.2.1 Indication shall be made on the control panel when this mode of operation is selected;
6.2.2 When the x-ray tube potential is equal to or greater than 51 kilovolts peak (kVp), the minimum exposure time for field emission equipment rated for pulse operation shall be equal to or less than a time interval equivalent to two pulses and the minimum exposure time for all other equipment shall be equal to or less than 1/60 second or a time interval required to deliver 5 milliampere-seconds (mAs), whichever is greater;
6.2.3 Either the product of peak x-ray tube potential, current, and exposure time shall be limited to not more than 60 kilowatt-seconds (kWs) per exposure or the product of x-ray tube current and exposure time shall be limited to not more than 600 mAs per exposure, except when the x-ray tube potential is less than 51 kVp, in which case the product of x-ray tube current and exposure time shall be limited to not more than 2,000 mAs per exposure; and
6.2.4 A visible signal shall indicate when an exposure has been terminated at the limits described in 6.2.3, and manual resetting shall be required before further automatically timed exposures can be made.
6.2.5 Accuracy. Deviation of technique factors from indicated values shall not exceed the limits given by the manufacturer.
6.2.5.1 Reproducibility. he following requirements shall apply when the equipment is operated on an adequate power supply as specified by the manufacturer:
6.2 Coefficient of variation. For any specific combination of selected technique factors, the estimated coefficient of variation of the air kerma shall be no greater than 0.05.
6.3 Measuring compliance. Determination of compliance shall be based on 10 consecutive measurements taken within a time period of 1 hour. Equipment manufactured after September 5, 1978, shall be subject to the additional requirement that all variable controls for technique factors shall be adjusted to alternate settings and reset to the test setting after each measurement. The percent line-voltage regulation shall be within ±1 of the mean value for all measurements. For equipment having automatic exposure controls, compliance shall be determined with a sufficient thickness of attenuating material in the useful beam such that the technique factors can be adjusted to provide individual exposures of a minimum of 12 pulses on field emission equipment rated for pulsed operation or no less than one-tenth second per exposure on all other equipment.
6.4 Linearity. The following requirements apply when the equipment is operated on a power supply as specified by the manufacturer in accordance with 21 CFR Part 1020 for any fixed x-ray tube potential within the range of 40 percent to 100 percent of the maximum rated.
6.4.1 Equipment having independent selection of x-ray tube current (mA). The average ratios of air kerma to the indicated milliampere-seconds product (mGy/mAs) obtained at any two consecutive tube current settings shall not differ by more than 0.10 times their sum. This is: ׀X1 – X2׀ ≤ 0.10(X1 + X2); where X1 and X2 are the average mGy/mAs values obtained at each of two consecutive mAs selector settings or at two settings differing by no more than a factor of 2 where the mAs selector provides continuous selection.
6.4.2 Equipment having selection of x-ray tube current-exposure time product (mAs). For equipment manufactured after May 3, 1994, the average ratios of air kerma to the indicated milliampere-seconds product (mGy/mAs) obtained at any two consecutive mAs selector settings shall not differ by more than 0.10 times their sum. This is: ׀X1 – X2׀ ≤ 0.10(X1 + X2); where X1 and X2 are the average mGy/mAs values obtained at each of two consecutive mAs selector settings or at two settings differing by no more than a factor of 2 where the mAs selector provides continuous selection.
6.4.3 Measuring compliance. Determination of compliance will be based on 10 exposures, made within 1 hour, at each of the two settings. These two settings may include any two focal spot sizes except where one is equal to or less than 0.45 mm and the other is greater than 0.45 mm. For purposes of this requirement, focal spot size is the focal spot size specified by the x-ray tube manufacturer. The percent line-voltage regulation shall be determined for each measurement. All values for percent line-voltage regulation at any one combination of technique factors shall be within ±1 of the mean value for all measurements at these technique factors.
6.5 Field limitation and alignment for mobile, portable, and stationary general purpose x-ray systems. Except when spot-film devices are in service, mobile, portable, and stationary general purpose radiographic x-ray systems shall meet the following requirements:
6.6 Variable x-ray field limitation. A means for stepless adjustment of the size of the x-ray field shall be provided. Each dimension of the minimum field size at an SID of 100 cm shall be equal to or less than 5 cm.
6.6.1 Visual definition.
6.6.1.1 Means for visually defining the perimeter of the x-ray field shall be provided. The total misalignment of the edges of the visually defined field with the respective edges of the x-ray field along either the length or width of the visually defined field shall not exceed 2 percent of the distance from the source to the center of the visually defined field when the surface upon which it appears is perpendicular to the axis of the x-ray beam.
6.6.1.2 When a light localizer is used to define the x-ray field, it shall provide an average illuminance of not less than 160 lux (15 footcandles) at 100 cm or at the maximum SID, whichever is less. The average illuminance shall be based on measurements made in the approximate center of each quadrant of the light field. Radiation therapy simulation systems are exempt from this requirement.
6.6.1.3 The edge of the light field at 100 cm or at the maximum SID, whichever is less, shall have a contrast ratio, corrected for ambient lighting, of not less than 4 in the case of beam-limiting devices designed for use on stationary equipment, and a contrast ratio of not less than 3 in the case of beam-limiting devices designed for use on mobile and portable equipment. The contrast ratio is defined as I1/I2, where I1 is the illuminance 3 mm from the edge of the light field toward the center of the field; and I2 is the illuminance 3 mm from the edge of the light field away from the center of the field. Compliance shall be determined with a measuring aperture of 1 mm.
6.7 Field indication and alignment on stationary general purpose x-ray equipment. Except when spot-film devices are in service, stationary general purpose x-ray systems shall meet the following requirements in addition to those prescribed in 6.5.
6.7.1 Means shall be provided to indicate when the axis of the x-ray beam is perpendicular to the plane of the image receptor, to align the center of the x-ray field with respect to the center of the image receptor to within 2 percent of the SID, and to indicate the SID to within 2 percent;
6.7.2 The beam-limiting device shall numerically indicate the field size in the plane of the image receptor to which it is adjusted;
6.7.3 Indication of field size dimensions and SIDs shall be specified in centimeters and/or inches and shall be such that aperture adjustments result in x-ray field dimensions in the plane of the image receptor which correspond to those indicated by the beam-limiting device to within 2 percent of the SID when the beam axis is indicated to be perpendicular to the plane of the image receptor; and
6.7.4 Compliance measurements will be made at discrete SIDs and image receptor dimensions in common clinical use (such as SIDs of 100, 150, and 200 cm and/or 36, 40, 48, 72 inches and nominal image receptor dimensions of 13, 18, 24, 30, 35, 40, and 43 cm and/or 5, 7, 8, 9, 10, 11, 12, 14, and 17 inches) or at any other specific dimensions at which the beam-limiting device or its associated diagnostic x-ray system is uniquely designed to operate.
6.8 Field limitation on radiographic x-ray equipment other than general purpose radiographic systems.
6.8.1 Equipment for use with intraoral image receptors. Radiographic equipment designed for use with an intraoral image receptor shall be provided with means to limit the x-ray beam such that:
6.8.1.1 If the minimum source-to-skin distance (SSD) is 18 cm or more, the x-ray field at the minimum SSD shall be containable in a circle having a diameter of no more than 7 cm; and
6.8.1.2 If the minimum SSD is less than 18 cm, the x-ray field at the minimum SSD shall be containable in a circle having a diameter of no more than 6 cm.
6.9 X-ray systems designed for one image receptor size. Radiographic equipment designed for only one image receptor size at a fixed SID shall be provided with means to limit the field at the plane of the image receptor to dimensions no greater than those of the image receptor, and to align the center of the x-ray field with the center of image receptor to within 2 percent of the SID, or shall be provided with means to both size and align the x-ray field such that the x-ray field at the plane of the image receptor does not extend beyond the edge of the image receptor.
6.10 Systems designed for mammography.
6.10.1 Radiographic systems designed only for mammography and general purpose radiography systems, when special attachments for mammography are in service, manufactured on or after November 1, 1977, and before September 30, 1999, shall be provided with means to limit the useful beam such that the x-ray field at the plane of the image receptor does not extend beyond any edge of the image receptor at any designated SID except the edge of the image receptor designed to be adjacent to the chest wall where the x-ray field may not extend beyond this edge by more than 2 percent of the SID. This requirement can be met with a system that performs as prescribed in 6.11.When the beam-limiting device and image receptor support device are designed to be used to immobilize the breast during a mammographic procedure and the SID may vary, the SID indication specified in 6.11 shall be the maximum SID for which the beam-limiting device or aperture is designed.
6.10.2 Mammographic beam-limiting devices manufactured on or after September 30, 1999, shall be provided with a means to limit the useful beam such that the x-ray field at the plane of the image receptor does not extend beyond any edge of the image receptor by more than 2 percent of the SID. This requirement can be met with a system that performs as prescribed in 6.11.For systems that allow changes in SID, the SID indication specified in 6.11 shall be the maximum SID for which the beam-limiting deviced or aperture is designed.
6.10.3 Each image receptor support device manufactured on or after November 1, 1977, intended for installation on a system designed for mammography shall have clear and permanent markings to indicate the maximum image receptor size for which it is designed.
6.11 Other x-ray systems. Radiographic systems not specifically covered in 6.5, 6.7, 6.8.1.2, 6.10, 6.18, and systems covered in 6.8.1, which are also designed for use with extraoral image receptors and when used with an extraoral image receptor, shall be provided with means to limit the x-ray field in the plane of the image receptor so that such field does not exceed each dimension of the image receptor by more than 2 percent of the SID, when the axis of the x-ray beam is perpendicular to the plane of the image receptor. In addition, means shall be provided to align the center of the x-ray field with the center of the image receptor to within 2 percent of the SID, or means shall be provided to both size and alignment the x-ray field such that the x-ray field at the plane of the image receptor does not extend beyond any edge of the image receptor. These requirements may be met with:
6.11.1 A system which performs in accordance with 6.5 and 6.7; or when alignment means are also provided, may be met with either;
6.11.2 An assortment of removable, fixed-aperture, beam-limiting devices sufficient to meet the requirement for each combination of image receptor size and SID for which the unit is designed. Each such device shall have clear and permanent markings to indicate the image receptor size and SID for which it is designed; or
6.11.3 A beam-limiting device having multiple fixed apertures sufficient to meet the requirement for each combination of image receptor size and SID for which the unit is designed. Permanent, clearly legible markings shall indicate the image receptor size and SID for which each aperture is designed and shall indicate which aperture is in position for use.
6.12 Positive beam limitation (PBL). The requirements of this subsection shall apply to radiographic systems which contain PBL.
6.12.1 Field size. When a PBL system is provided, it shall prevent x-ray production when:
6.12.1.1 Either the length or width of the x-ray field in the plane of the image receptor differs from the corresponding image receptor dimension by more than 3 percent of the SID; or
6.12.1.2 The sum of the length and width differences stated in 6.12.1.1 without regard to sign exceeds 4 percent of the SID.
6.13.1.3 The beam-limiting device is at an SID for which PBL is not designed for sizing.
6.13 Conditions for PBL. When provided, the PBL system shall function as described in 6.12.1 whenever all the following conditions are met:
6.13.1 The image receptor is inserted into a permanently mounted cassette holder;
6.13.2 The image receptor length and width are less than 50 cm;
6.13.3 The x-ray beam axis is within ±3 degrees of vertical and the SID is 90 cm to 130 cm inclusive; or the x-ray beam axis is within ±3 degrees of horizontal and the SID is 90 cm to 205 cm inclusive;
6.13.4 The x-ray beam axis is perpendicular to the plane of the image receptor to within ±3 degrees; and
6.13.5 Neither tomographic nor stereoscopic radiography is being performed.
6.14 Measuring compliance. Compliance with the requirements of 6.12.1. shall be determined when the equipment indicates that the beam axis is perpendicular to the plane of the image receptor and the provisions of 6.13 are met. Compliance shall be determined no sooner than 5 second after insertion of the image receptor.
6.15 Operator initiated undersizing. The PBL system shall be capable of operating such that, at the discretion of the operator, the size of the field may be made smaller than the size of the image receptor through stepless adjustment of the field size. Each dimension of the minimum field size at an SID of 100 cm shall be equal to or less than 5 cm. Return to PBL function as described in 6.12.1 shall occur automatically upon any change of image receptor size or SID.
6.16 Override of PBL. A capability may be provided for overriding PBL in case of system failure and for servicing the system. This override may be for all SIDs and image receptor sizes. A key shall be required for any override capability that is accessible to the operator. It shall not be possible to remove the key while PBL is overridden. Each such key switch or key shall be clearly and durably labeled as follows:
6.17 For X-Ray Field Limitation System Failure
6.17.1 The override capability is considered accessible to the operator if it is referenced in the operator’s manual or in other material intended for the operator or if its location is such that the operator would consider it part of the operational controls.
6.18 Field limitation and alignment for spot-film devices. The following requirements shall apply to spot-film devices, except when the spot-film device is provided for use with a radiation therapy simulation system:
6.18.1 Means shall be provided between the source and the patient for adjustment of the x-ray field size in the plane of the image receptor to the size of that portion of the image receptor which has been selected on the spot-film selector. Such adjustment shall be accomplished automatically when the x-ray field size in the plane of the image receptor is greater than the selected portion of the image receptor. If the x-ray field size is less than the size of the selected portion of the image receptor, the field size shall not open automatically to the size of the selected portion of the image receptor unless the operator has selected that mode of operation.
6.18.2 Neither the length nor width of the x-ray field in the plane of the image receptor shall differ from the corresponding dimensions of the selected portion of the image receptor by more than 3 percent of the SID when adjusted for full coverage of the selected portion of the image receptor. The sum, without regard to sign, of the length and width differences shall not exceed 4 percent of the SID. On spot film devices manufactured after February 25, 1978, if the angle between the plane of the image receptor and beam axis is variable, means shall be provided to indicate when the axis of the x-ray beam is perpendicular to the plane of the image receptor, and compliance shall be determined with the beam axis indicated to be perpendicular to the plane of the image receptor.
6.18.3 The center of the x-ray field in the plane of the image receptor shall be aligned with the center of the selected portion of the image receptor to within 2 percent of the SID.
6.18.4 Means shall be provided to reduce the x-ray field size in the plane of the image receptor to a size smaller than the selected portion of the image receptor such that:
6.18.4.1 For spot-film devices used on fixed-SID fluoroscopic systems which are not required to, and do not provide stepless adjustment of the x-ray field, the minimum field size, at the greatest SID, does not exceed 125 square cm; or
6.18.4.2 For spot-film devices used on fluoroscopic systems that have a variable SID and/or stepless adjustment of the field size, the minimum field size, at the greatest SID, shall be containable in a square of 5 cm by 5 cm.
6.19 A capability may be provided for overriding the automatic x-ray field size adjustment in case of system failure. If it is so provided, a signal visible at the fluoroscopist’s position shall indicate whenever the automatic x-ray field size adjustment override is engaged. Each such system failure override switch shall be clearly labeled as follows:
6.20 For X-ray Field Limitation System Failure
6.20.1 Source-skin distance.
6.20.1.1 X-ray systems designed for use with an intraoral image receptor shall be provided with means to limit the source-skin distance to not less than:
6.20.1.1.1 Eighteen cm if operable above 50 kVp; or
6.20.1.1.2 Ten cm if not operable above 50 kVp.
6.21 Mobile and portable x-ray systems other than dental shall be provided with means to limit the source-skin distance to not less than 30 cm. (See Appendix B for dental systems)
6.22 Beam-on indicators. The x-ray control shall provide visual indication whenever x-rays are produced. In addition, a signal audible to the operator shall indicate that the exposure has terminated.
6.23 Reserved.
6.24 Radiation from capacitor energy storage equipment. Radiation emitted from the x-ray tube shall not exceed:
6.24.1 An air kerma of 0.26 microGy (vice 0.03 mR exposure) in 1 minute at 5 cm from any accessible surface of the diagnostic source assembly, with the beam-limiting device fully open, the system fully charged, and the exposure switch, timer, or any discharge mechanism not activated. Compliance shall be determined by measurements averaged over an area of 100 square cm, with no linear dimensions greater than 20 cm: and
6.24.2 An air kerma of 0.88 mGy (vice 100 mR exposure) in one hour at 100 cm from the x-ray source, with beam-limiting device fully open, when the system is discharged through the x-ray tube either manually or automatically by use of a discharge switch or deactivation of the input power. Compliance shall be determined by measurements of the maximum air kerma per discharge multiplied by the total number of discharges in 1 hour (duty cycle). The measurements shall be averaged over an area of 100 square cm with no linear dimension greater than 20 cm.
6.25 Primary protective barrier for mammography x-ray systems.
6.25.1 For x-ray systems manufactured after September 5, 1978, and before September 30, 1999, which are designed only for mammography, the transmission of the primary beam through any image receptor support provided with the system shall be limited such that the air kerma 5 cm from any accessible surface beyond the plane of the image receptor supporting device does not exceed 0.88 microGy (vice 0.1 mR exposure) for each activation of the tube.
6.25.2 For mammographic x-ray systems manufactured on or after September 30, 1999:
6.25.2.1 At any SID where exposures can be made, the image receptor support device shall provide a primary protective barrier that intercepts the cross section of the useful beam along every direction except at the chest wall edge.
6.25.2.2 The x-ray system shall not permit exposure unless the appropriate barrier is in place to intercept the useful beam as required in 6.25.2.1.
6.25.2.3 The transmission of the useful beam through the primary protective barrier shall be limited such that the air kerma 5 cm from any accessible surface beyond the plane of the primary protective barrier does not exceed 0.88 microGy (vice 0.1 mR exposure) for each activation of the tube.
6.26 Compliance with the requirements of 6.25.1 and 6.25.2.3 for transmission shall be determined with the x-ray system operated at the minimum SID for which it is designed, at maximum rated peak tube potential, at the maximum rated product of x-ray tube current and exposure time (mAs) for the maximum rated peak tube potential, and by measurements averaged over an area of 100 square cm with no linear dimension greater than 20 cm. The sensitive volume of the radiation measuring instrument shall not be positioned beyond the edge of the primary protective barrier along the chest wall side.
6.27 Beam Limitation, Except Mammographic Systems. The useful beam shall be limited to the area of clinical interest. This shall be deemed to have been met if a positive beam limiting device meeting manufacturer's specifications and the requirements of 6.12 have been properly used or if evidence of collimation is shown on at least three sides or three corners of the film (for example, projections from the shutters of the collimator, cone cutting at the corners, or borders at the film's edge).
6.28 Radiation Exposure Control.
6.28.1 Exposure Initiation. Means shall be provided to initiate the radiation exposure by a deliberate action on the part of the operator, such as the depression of a switch. Radiation exposure shall not be initiated without such an action. In addition, it shall not be possible to initiate an exposure when the timer is set to a "zero" or "off" position if either position is provided.
6.28.2 Exposure Indication. Means shall be provided for visual indication observable at or from the operator's protected position whenever x-rays are produced. In addition, a signal audible to the operator shall indicate that the exposure has terminated.
6.28.3 Operator Protection, Except Veterinary Systems.
6.28.3.1 Stationary Systems. Stationary x-ray systems shall be required to have the x-ray control permanently mounted in a protected area so that the operator is required to remain in that protected area during the entire exposure.
6.28.3.2 Mobile and Portable Systems. Mobile and portable x-ray systems which are:
6.28.3.2.1 Used continuously for greater than one week in the same location, i.e., a room or suite, shall meet the requirements of 6.28.3.1
6.28.3.2.2 Used for less than one week at the same location shall be provided with either a protective barrier at least 2 meters (6.5 feet) high for operator protection during exposures, or means shall be provided to allow the operator to be at least 2.7 meters (9 feet) from the tube housing assembly during the exposure.
6.29 Operator Protection for Veterinary Systems. All stationary, mobile or portable x-ray systems used for veterinary work shall be provided with either a 2 meter (6.5 feet) high protective barrier for operator protection during exposures, or shall be provided with means to allow the operator to be at least 2.7 meters (9 feet) from the tube housing assembly during exposures. Refer to Appendix B for hand-held intraoral dental radiographic units used in veterinary practice.
6.30 Tube Stands for Portable X-Ray Systems. A tube stand or other mechanical support shall be used for portable x-ray systems, so that the x-ray tube housing assembly need not be hand-held during exposures.
In addition to the applicable provisions of 3.0, 4.0, 6.0, the requirements of 7.0 apply to x-ray equipment and associated facilities used for dental intraoral radiography. Requirements for extraoral dental radiographic systems are covered in 6.0.
7.1 Radiation Exposure Control. Means shall be provided to initiate the radiation exposure by a deliberate action on the part of the operator, such as the depression of a switch. Radiation exposure shall not be initiated without such an action.
7.1.1 Exposure Control Location and Operator Protection.
7.2 Stationary x‑ray systems shall be required to have the x‑ray exposure control permanently mounted in a protected area, so that the operator is required to remain in that protected area during the entire exposure; and
7.3 Mobile and portable x‑ray systems which are:
7.3.1 Used for greater than one week in the same location, i.e., a room or suite, shall meet the requirements of 7.1;
7.3.2 Used for less than one week in the same location shall be provided with either a protective barrier at least 2 meters (6.5 feet) high for operator protection, or means to allow the operator to be at least 2.7 meters (9 feet) from the tube housing assembly while making exposures.
7.4 kVp Limitations. Dental x-ray machines with a nominal fixed kVp of less than 50 kVp shall not be used to make diagnostic dental radiographs of humans.
7.5 Administrative Controls.
7.5.1 Patient and film holding devices shall be used when the techniques permit.
7.5.2 The tube housing and the PID for a permanently mounted intraoral dental system shall not be hand-held during an exposure. Appendix B specifies requirements for the use of intraoral dental radiographic units designed to be hand-held during patient examination.
7.5.3 Dental fluoroscopy without image intensification shall not be used.
8.1 Definitions. In addition to the definitions provided in A.2 and 2.0 of these regulations, the following definitions shall be applicable to 8.0
"Computed tomography dose index" (CTDI) means the integral of the dose profile along a line perpendicular to the tomographic plane divided by the product of the nominal tomographic section thickness and the number of tomograms produced in a single scan, that is:
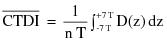
z = Position along a line perpendicular to the tomographic plane;
D(z) = Dose at position z;
T = Nominal tomographic section thickness;
n = Number of tomograms produced in a single scan.
This definition assumes that the dose profile is centered around z=0 and that, for a multiple tomogram system, the scan increment between adjacent scans is nT.
"Contrast scale" means the change in the linear attenuation coefficient per CTN relative to water, that is:
where:
 = Linear attenuation coefficient of the material of interest;
= Linear attenuation coefficient of the material of interest;
 = Linear attenuation coefficient of water;
= Linear attenuation coefficient of water;
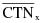 = of the material of interest;
= of the material of interest;
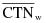 = of water.
= of water.
"CT conditions of operation" means all selectable parameters governing the operation of a CT x-ray system including nominal tomographic section thickness, filtration, and the technique factors as defined in 2.0.
"CT dosimetry phantom" means the phantom used for determination of the dose delivered by a CT x-ray system. The phantom shall be a right circular cylinder of polymethl-methacrylate of density 1.19±0.01 grams per cubic centimeter. The phantom shall be at least 14 centimeters in length and shall have diameters of 32.0 centimeters for testing any CT system designed to image any section of the body (whole body scanners) and 16.0 centimeters for any system designed to image the head (head scanners) or for any whole body scanner operated in the head scanning mode. The phantom shall provide means for the placement of a dosimeter(s) along its axis of rotation and along a line parallel to the axis of rotation 1.0 centimeter from the outer surface and within the phantom. Means for the placement of a dosimeter(s) or alignment device at other locations may be provided for convenience. Any effect on the doses measured due to the removal of phantom material to accommodate dosimeters shall be accounted for through appropriate corrections to the reported data or included in the statement of maximum deviation for the values obtained using the phantom.
"CT Number" means the number used to represent the x-ray attenuation associated with each elemental area of the CT image:
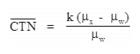
where:
k = A constant, a normal value of 1,000 when the Houndsfield scale of CTN is used;
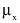 = Linear attenuation coefficient of the material of interest;
= Linear attenuation coefficient of the material of interest;
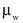 = Linear attenuation coefficient of water.
= Linear attenuation coefficient of water.
"Dose profile" means the dose as a function of position along a line.
"Modulation transfer function" means the modulus of the Fourier transform of the impulse response of the system.
"Multiple tomogram system" means a computed tomography x-ray system which obtains x-ray transmission data simultaneously during a single scan to produce more than one tomogram.
"Noise" means the standard deviation of the fluctuations in CTN expressed as a percentage of the attenuation coefficient of water. Its estimate (Sn) is calculated using the following expression:
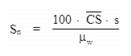
where:
 = Linear attenuation coefficient of the material of interest.
= Linear attenuation coefficient of the material of interest.
 = Linear attenuation coefficient of water.
= Linear attenuation coefficient of water.
 = Standard deviation of the CTN of picture elements in a specified area of the CT image.
= Standard deviation of the CTN of picture elements in a specified area of the CT image.
"Nominal tomographic section thickness" means the full width at half-maximum of the sensitivity profile taken at the center of the cross-sectional volume over which x-ray transmission data are collected.
"Picture element" means an elemental area of a tomogram.
"Reference plane" means a plane which is displaced from and parallel to the tomographic plane.
"Remanufacturing" means modifying a CT system in such a way that the resulting dose and imaging performance become substantially equivalent to any CT x-ray system manufactured by the original manufacturer on or after November 29, 1984. Any reference in this subsection to “manufacture,” “manufacturer,” or “manufacturing” includes remanufacture, remanufacturing, respectively.
"Scan" means the complete process of collecting x-ray transmission data for the production of a tomogram. Data can be collected simultaneously during a single scan for the production of one or more tomograms.
"Scan increment" means the amount of relative displacement of the patient with respect to the CT x-ray system between successive scans measured along the direction of such displacement.
"Scan sequence" means a pre-selected set of two or more scans performed consecutively under pre-selected CT conditions of operation.
"Sensitivity profile" means the relative response of the CT x-ray system as a function of position along a line perpendicular to the tomographic plane.
"Single tomogram system" means a CT x-ray system which obtains x-ray transmission data during a scan to produce a single tomogram.
"Tomographic plane" means that geometric plane which the manufacturer identified as corresponding to the output tomogram.
"Tomographic section" means the volume of an object whose x-ray attenuation properties are imaged in a tomogram.
8.2 Requirements for Equipment.
8.2.1 Termination of Exposure.
8.2.1.1 Means shall be provided to terminate the x-ray exposure automatically by either de-energizing the x-ray source or shuttering the x-ray beam in the event of equipment failure affecting data collection. Such termination shall occur within an interval that limits the total scan time to no more than 110 percent of its preset value through the use of either a backup timer or devices which monitor equipment function.
8.2.1.2 A visible signal shall indicate when the x-ray exposure has been terminated through the means required by Subdivision 8.2.1.1.
8.2.1.3 The operator shall be able to terminate the x-ray exposure at any time during a scan, or series of scans under CT x-ray system control, of greater than one-half second duration.
8.3 Tomographic Plane Indication and Alignment.
8.3.1 For any single tomogram system, means shall be provided to permit visual determination of the tomographic plane or a reference plane offset from the tomographic plane.
8.3.2 For any multiple tomogram system, means shall be provided to permit visual determination of the location of a reference plane. This reference plane can be offset from the location of the tomographic planes.
8.3.3 If a device using a light source is used to satisfy the requirements of Subdivisions 8.3.1 or 8.3.2, the light source shall provide illumination levels sufficient to permit visual determination of the location of the tomographic plane or reference plane under ambient light conditions of up to 500 lux.
8.4 Beam-On and Shutter Status Indicators and Control Switches.
8.4.1 The CT x-ray control and gantry shall provide visual indication whenever x-rays are produced and, if applicable, whether the shutter is open or closed.
8.4.2 Each emergency button or switch shall be clearly labeled as to its function.
8.5 Indication of CT Conditions of Operation. The CT x-ray system shall be designed such that the CT conditions of operation to be used during a scan or a scan sequence shall be indicated prior to the initiation of a scan or a scan sequence. On equipment having all or some of these conditions of operation at fixed values, this requirement may be met by permanent markings. Indication of CT conditions of operation shall be visible from any position from which scan initiation is possible.
8.6 Extraneous Radiation. When data are not being collected for image production, the radiation adjacent to the tube port shall not exceed that permitted by 4.3.
8.7 Maximum Surface CTDI Identification. The angular position where the maximum surface CTDI occurs shall be identified to allow for reproducible positioning of a CT dosimetry phantom.
8.8 Additional Requirements Applicable to CT X-Ray Systems Containing a Gantry Manufactured After September 3, 1985.
8.8.1 The total error in the indicated location of the tomographic plane or reference plane shall not exceed 5 millimeters.
8.8.2 If the x-ray production period is less than one-half second, the indication of x-ray production shall be actuated for at least one-half second. Indicators at or near the gantry shall be discernible from any point external to the patient opening where insertion of any part of the human body into the primary beam is possible.
8.8.3 The deviation of indicated scan increment versus actual increment shall not exceed plus or minus 1 millimeter with any mass from 0 to 100 kilograms resting on the support device. The patient support device shall be incremented from a typical starting position to the maximum incremented distance or 30 centimeters, whichever is less, and then returned to the starting position. Measurement of actual versus indicated scan increment may be taken anywhere along this travel.
8.8.4 Premature termination of the x-ray exposure by the operator shall necessitate resetting of the CT conditions of operation prior to the initiation of another scan.
8.9 Facility Design Requirements.
8.9.1 Aural Communication. Provision shall be made for two-way aural communication between the patient and the operator at the control panel.
8.9.2 Viewing Systems.
8.9.2.1 Windows, mirrors, closed-circuit television, or an equivalent shall be provided to permit continuous observation of the patient during irradiation and shall be so located that the operator can observe the patient from the control panel.
8.9.2.2 When the primary viewing system is by electronic means, an alternate viewing system (which may be electronic) shall be available for use in the event of failure of the primary viewing system.
8.10 Surveys, Calibrations, Spot Checks, and Operating Procedures.
8.11 Surveys.
8.11.1 All CT x-ray systems installed after [insert the effective date of the regulations] and those systems not previously surveyed shall have a survey made by, or under the direction of, a qualified medical physicist. In addition, such surveys shall be done after any change in the facility or equipment which might cause a significant increase in radiation hazard.
8.11.2 The registrant [licensee] shall obtain a written report of the survey from the qualified medical physicist, and a copy of the report shall be made available to the Agency upon request.
8.12 Radiation Calibrations.
8.12.1 The calibration of the radiation output of the CT x-ray system shall be performed by, or under the direction of, a qualified medical physicist who is physically present at the facility during such calibration.
8.12.2 The calibration of a CT x-ray system shall be performed after initial installation and before use on human patients, annually or at intervals specified by a qualified medical physicist, and after any change or replacement of components which, in the opinion of the qualified medical physicist, could cause a change in the radiation output.
8.12.3 The calibration of the radiation output of a CT x-ray system shall be performed with a calibrated dosimetry system. The calibration of such system shall be traceable to a national standard. The dosimetry system shall have been calibrated within the preceding 2 years.
8.12.4 CT dosimetry phantom(s) shall be used in determining the radiation output of a CT x-ray system. Such phantom(s) shall meet the following specifications and conditions of use:
8.12.4.1 CT dosimetry phantom(s) shall be right circular cylinders of polymethyl methacrylate of density 1.19 plus or minus 0.01 grams per cubic centimeter. The phantom(s) shall be at least 14 centimeters in length and shall have diameters of 32.0 centimeters for testing CT x-ray systems designed to image any section of the body and 16.0 centimeters for systems designed to image the head or for whole body scanners operated in the head scanning mode;
8.12.4.2 CT dosimetry phantom(s) shall provide means for the placement of a dosimeter(s) along the axis of rotation and along a line parallel to the axis of rotation 1.0 centimeter from the outer surface and within the phantom. Means for the placement of dosimeters or alignment devices at other locations may be provided;
8.12.4.3 Any effects on the doses measured due to the removal of phantom material to accommodate dosimeters shall be accounted for through appropriate corrections to the reported data or included in the statement of maximum deviation for the values obtained using the phantom;
8.12.4.4 All dose measurements shall be performed with the CT dosimetry phantom placed on the patient couch or support device without additional attenuation materials present.
8.12.5 The calibration shall be required for each type of head, body, or whole-body scan performed at the facility.
8.12.6 Calibration shall meet the following requirements:
8.12.6.1 The dose profile along the center axis of the CT dosimetry phantom for the minimum, maximum, and midrange values of the nominal tomographic section thickness used by the registrant shall be measurable. Where less than 3 nominal tomographic thicknesses can be selected, the dose profile determination shall be performed for each available nominal tomographic section thickness;
8.12.6.2 The CTDI2/ along the two axes specified in Subdivision 8.12.4.2 shall be measured. The CT dosimetry phantom shall be oriented so that the measurement point 1.0 centimeter from the outer surface and within the phantom is in the same angular position within the gantry as the point of maximum surface CTDI identified. The CT conditions of operation shall correspond to typical values used by the registrant;
8.12.6.3 The spot checks specified in 8.13 shall be made.
8.12.7 Calibration procedures shall be in writing. Records of calibrations performed shall be maintained for inspection by the Agency.
8.13 Spot Checks.
8.13.1 The spot-check procedures shall be in writing and shall have been developed by a qualified medical physicist.
8.13.2 The spot-check procedures shall incorporate the use of a CT dosimetry phantom which has a capability of providing an indication of contrast scale, noise, nominal tomographic section thickness, the resolution capability of the system for low and high contrast objects, and measuring the mean CTN for water or other reference material.
8.13.3 All spot checks shall be included in the calibration required by 8.11.2. and at time intervals and under system conditions specified by a qualified medical physicist.
8.13.4 Spot checks shall include acquisition of images obtained with the CT dosimetry phantom(s) using the same processing mode and CT conditions of operation as are used to perform calibrations required by 8.11.2. The images shall be retained, until a new calibration is performed, in two forms as follows:
8.13.4.1 Photographic copies of the images obtained from the image display device; and
8.13.4.2 Images stored in digital form on a storage medium compatible with the CT x-ray system.
8.13.5 Written records of the spot checks performed shall be maintained for inspection by the Agency.
8.14 Operating Procedures.
8.14.1 The CT x-ray system shall not be operated except by an individual who has been specifically trained in its operation.
8.14.2 Information shall be available at the control panel regarding the operation and calibration of the system. Such information shall include the following:
8.14.2.1 Dates of the latest calibration and spot checks and the location within the facility where the results of those tests may be obtained;
8.14.2.2 Instructions on the use of the CT dosimetry phantom(s) including a schedule of spot checks appropriate for the system, allowable variations for the indicated parameters, and the results of at least the most recent spot checks conducted on the system;
8.14.2.3 The distance in millimeters between the tomographic plane and the reference plane if a reference plane is utilized; and
8.14.2.4 A current technique chart available at the control panel which specifies for each routine examination the CT conditions of operation and the number of scans per examination.
8.14.3 If the calibration or spot check of the CT x-ray system identifies that a system operating parameter has exceeded a tolerance established by the qualified medical physicist, use of the CT x-ray system on patients shall be limited to those uses permitted by established written instructions of the qualified medical physicist.
9.1 Requirements for Certification.
9.1.1 Only x-ray systems, pursuant to the Mammography Quality Standards Reauthorization Act of 1998, Public Law 105-248, and 21 C.F.R. Part 900, shall be used for screening and diagnostic mammography.
9.1.2 A facility performing mammography shall have a valid certificate issued by the U.S. Department of Health and Human Services, pursuant to the Mammography Quality Standards Reauthorization Act of 1998, Public Law 105-248, and 21 C.F.R. Part 900.
9.1.3 A facility performing mammography shall ensure that the additional mammography activities of processing the x-ray film, interpreting the image, and maintaining viewing conditions, wherever performed, meet all quality standards pursuant to the Mammography Quality Standards Reauthorization Act of 1998, Public Law 105-248, and 21 C.F.R. Part 900.
[Current] federal regulations, 21 CFR Part 900, [dated] referring to states with certifying authority, is incorporated in its entirety in Part F.
11.1 Bone densitometry systems shall be:
11.1.1 Certified by the manufacturer pursuant to the Medical Device Act and Subchapter C – Electronic Product Radiation Control (EPRC) of Chapter V of the Federal Food, Drug and Cosmetic Act;
11.1.2 Registered [licensed] in accordance with Part B of these regulations; and
11.1.3 Maintained and operated in accordance with the manufacturer’s specifications.
11.2 Equipment Requirements. Systems with stepless collimators shall be provided with means to both size and align the x-ray field such that the x-ray field at the plane of the image receptor does not extend beyond 2 percent of the SID.
11.3 Operators of bone densitometry systems shall be:
11.3.1 Licensed, certified, or permitted as a radiologic technologist [or technician] [by the Agency]; or
11.3.2 Licensed as a practitioner of the healing arts; or
11.3.3 Permitted, certified, or approved [by the Agency] as a bone densitometry operator[; or
11.3.4 Complete a training course on bone densitometry which is approved by the Agency. The training course shall include:
11.3.4.1 Basic radiation protection;
11.3.4.2 Operating procedures for bone densitometry systems, to include use of various system functions, safety, and maintenance; and
11.3.4.3 Patient positioning for the types of examinations performed.]
11.4 During the operation of any bone densitometry system:
11.4.1 The operator, ancillary personnel, and members of the general public shall be positioned at least one meter from the patient and bone densitometry system during the examination.
[11.4.2 The operator shall advise the patient that the bone densitometry examination is a type of x-ray procedure.]
11.5 The registrant [licensee] shall keep maintenance records for bone densitometry systems as prescribed by 11.1.3. These records shall be maintained for inspection by the Agency [[insert Agency recordkeeping timelines as appropriate]].
11.6 Bone densitometry on human patients shall be conducted only:
11.6.1 Under a prescription of a licensed practitioner of the healing arts; or
11.6.2 Under a screening program approved by the Agency.
11.7 Any person proposing to conduct a bone densitometry screening program shall submit the information outlined in Appendix A of this Part with the exception of g., h., i., j., k., and m., and include the name and address of the individual who will interpret the screening results.
12.1 All registrants [licensees] of diagnostic x-ray imaging equipment shall establish and maintain a quality assurance program consisting of quality control assessments addressing at least the following items:
12.1.1 Administration.
12.1.1.1 Written standard operating procedures on radiation protection and the practice of radiologic technology reviewed and updated annually by management;
12.1.1.2 Employee review and written acknowledgment of standard operating procedures and policies on radiation protection and the practice of radiologic technology;
12.1.1.3 Credentialing of practitioners, medical physicists, and x-ray equipment operators; and
12.1.1.4 Record retention in accordance with state statutes, regulations, but in no case less than three years.
12.1.2 Film Processing equipment.
12.1.2.1 Compliance with Section 3.0;
12.1.2.2 Film processor performance to include medium density, density difference, and base + fog;
12.1.2.3 Darkroom fog;
12.1.3 Radiographic equipment.
12.1.3.1 Compliance with performance standards in Sections 4.0 and 6.0;
12.1.3.2 Entrance skin exposure rates of selected patient examinations;
12.1.3.3 Image printing and viewing equipment;
12.1.3.4 Measurement of low and high contrast resolution; and
12.1.3.5 Radiation protection.
12.1.4 Fluoroscopic equipment.
12.1.4.1 Compliance with performance standards in Sections 4.0 and 5.0;
12.1.4.2 Entrance skin exposure rates of selected patient examinations;
12.1.4.3 Image printing and viewing equipment;
12.1.4.4 Measurement of low and high contrast resolution; and
12.1.4.5 Radiation protection.
12.1.5 Computerized tomography equipment.
12.1.5.1 Compliance with performance standards in Section 8.0;
12.1.5.2 CT number;
12.1.5.3 Low contrast and high contrast resolution;
12.1.5.4 Dosimetry of selected patient examinations to include pediatric patients if applicable;
12.1.5.5 Image printing and viewing equipment; and
12.1.5.6 Radiation protection.
12.1.6 Bone densitometry equipment.
12.1.6.1 Compliance with requirements in Section 11.0
12.1.7 Structural shielding for new facilities with x-ray equipment.
12.1.7.1 Pre-construction shielding design and evaluation; and
12.2 Post-construction radiation protection survey.
12.2.1 Structural shielding for modifying use or equipment in existing facility.
12.2.1.1 Re-evaluation of shielding design; and
12.2.1.2 Post-modification radiation protection survey.
12.3 The registrant [licensee] shall assign qualified personnel to fully implement the quality assurance program.
12.4 Quality control assessments for Section 12.1.1 may be assigned to qualified personnel who possess the requisite training and/or experience.
12.5 Quality control assessments for Section 12.1.1 shall be conducted by, or under the direction of, a qualified medical physicist.
12.6 The registrant [licensee] and/or qualified medical physicist shall determine the frequency of quality control tests.
12.7 The quality assurance program shall be in written form and available for review by the Agency.
12.8 Equipment used for compliance with the provisions of this section shall be properly calibrated and maintained in accordance with accepted professional standards.
This Section does not pertain to quality assurance for mammography equipment.
PART F
APPENDIX A
INFORMATION TO BE SUBMITTED BY PERSONS PROPOSING TO CONDUCT HEALING ARTS SCREENING
Persons requesting that the Agency approve a healing arts screening program shall submit the following information and evaluation:
1. Name and address of the applicant and, where applicable, the names and addresses of agents within this State;
2. Diseases or conditions for which the x‑ray examinations are to be used in diagnoses;
3. A description of the x‑ray examinations proposed in the screening program i.e., type and number of views;
4. Description of the population to be examined in the screening program, i.e., age range, sex, physical condition, and other appropriate information;
5. An evaluation of any known alternate methods not involving ionizing radiation that could achieve the goals of the screening program and why these methods are not used instead of the x‑ray examinations;
6. An evaluation by a qualified medical physicist of the x‑ray system(s) to be used in the screening program. The evaluation shall include the following:
a. Documentation that such system(s) satisfy all requirements of these regulations;
b. measurement of patient exposures from the x-ray examinations to be performed;
7. A description of the diagnostic x-ray quality control program;]
8. A copy of the technique chart for the x‑ray examination procedures to be used;
9. The qualifications of each individual who will be operating the x‑ray system(s);
10. The qualifications of the individual who will be supervising the operators of the x‑ray system(s). The extent of supervision and the method of work performance evaluation shall be specified;
11. The name and address of the practitioner licensed in the state who will interpret the radiograph(s);
12. Procedures to be used in advising the individuals screened and their practitioner of the healing arts or health care provider of the results of the screening procedure and any further medical needs indicated;
13. Procedures for the retention or disposition of the radiographs and other records pertaining to the x‑ray examinations;
14. Frequency of screening of individuals; and
15. The duration of the screening program.
PART F
APPENDIX B
HAND-HELD INTRAORAL DENTAL RADIOGRAPHIC UNIT REQUIREMENTS FOR USE
This appendix list the required information/documentation one must provide when requesting a variance for the use of a hand held unit. Such variance shall be specific to the particular facility location only.
The use of hand-held intraoral dental radiographic units must be approved as a variance by the Authority on Radiation Protection Board. Applications for variance must specify the conditions of use and precautions that will be put in place to assure safety and health protection equivalent to that of a stationary device.
The Agency may specify any of, but not be limited to, the following requirements for intraoral dental radiographic units designed to be operated as a hand-held unit:
1. For all uses:
a. The use of hand held intraoral dental radiographic units must be approved by the agency prior to use.
b. Operators of hand-held intraoral dental radiographic units shall be specifically trained to operate such equipment.
c. When operating a hand-held intraoral dental radiographic unit, operators shall wear a lead apron and thyroid collar, unless otherwise authorized by the Agency or a qualified health or medical physicist.
d. A hand-held intraoral dental radiographic unit shall be held without any motion during a patient examination. A tube stand may be utilized to immobilize a hand-held intraoral dental radiographic unit during patient examination.
e. Unless otherwise authorized by the Agency, a hand-held intraoral dental radiographic unit shall be used with a secondary radiation block.
f. The operator shall ensure there are no bystanders within a radius of at least six feet from the patient being examined with a hand-held intraoral radiographic unit.
2. Additional requirements for operatories in permanent facilities:
a. Hand-held intraoral dental radiographic units shall be used for patient examinations in dental operatories that meet the structural shielding requirements specified by the Agency or by a qualified health or medical physicist.
b. Hand-held intraoral dental radiographic units shall not be used for patient examinations in hallways and waiting rooms.
3. The following sections prohibit the use of hand held devices:
a. 3.1.9.3. Portable or mobile x-ray equipment shall be used only for examinations where it is impractical to transfer the patient(s) to a stationary x-ray installation. The use of hand held devices must be approved by the Agency utilizing the criteria in Appendix B.
b. 6.28.3.2. Mobile and Portable Systems. Mobile and portable x-ray systems which are:
6.28.3.2.1 Used continuously for greater than one week in the same location, i.e., a room or suite, shall meet the requirements of 6.28.3.1;
6.28.3.2.2 Used for less than one week at the same location shall be provided with either a protective barrier at least 2 meters (6.5 feet) high for operator protection during exposures, or means shall be provided to allow the operator to be at least 2.7 meters (9 feet) from the tube housing assembly during the exposure.
c. 6.30 Tube Stands for Portable X-Ray Systems. A tube stand or other mechanical support shall be used for portable x-ray systems, so that the x-ray tube housing assembly need not be hand-held during exposures.
d. 7.5.2 The tube housing and the PID for a permanently mounted intraoral dental system shall not be hand-held during an exposure. Appendix B specifies requirements for the use of intraoral dental radiographic units designed to be hand-held during patient examination.
Part G
This part provides for the licensing of radioactive material, for purposes of protecting the public health and safety. No person shall receive, possess, use, transfer, sell, own or acquire radioactive material except as authorized in a specific or general license per the U.S. Nuclear Regulatory Commission (NRC), in accordance with Title 10 – Code of Federal Regulations. Primary radioactive material licensing and enforcement authority was transferred to the NRC in 2007, pursuant to the Federal Energy Policy Act of 2005. However, radioactive material facilities must be registered with the State of Delaware in accordance with Part B of these regulations.
Part K
1.1 No person shall use/operate a source of radiation or radiation facility who does not possess a valid certificate, license or registration issued or renewed to that person by the Agency in accordance with Regulation 4481, sections 4.0, 7.0 and 9.0; Part C, sections 1.0 and 33; Regulation 4466, section 7.0 (previously referenced as DRCR B.4, B.7, B.9, C.1, C.33 or RTCR VII A) of the regulations. Only a person who complies with the requirements of the regulations shall be entitled to receive or retain such a certificate, license or registration.
1.2 The owner/manager of a radiation facility shall designate a Radiation Safety Officer (RSO) in accordance with the regulations.
1.3 All facility licenses, registrations and Radiation Technologist/Technician Certificates must be posted in a conspicuous location.
1.4 Any action taken by the Agency against an applicant or certificate, license or registration holder may be appealed to the Authority on Radiation Protection.
“Applicant” means a Person seeking a certificate, license or registration issued under the provisions of the Act and the requirements of the regulations.
“Certificate” is an official document issued by the Agency which authorizes a person to perform a specified radiation activity.
“Exemption” means an exclusion from a regulatory requirement granted by the Agency or Authority. When the exclusion is based on a national standard or similar documented and publicly available information, the Agency may grant it. Otherwise, the exemption shall be referred to the Authority for consideration.
“Hearing” means a proceeding to examine an application or other matter before the Authority in order to adjudicate rights, duties, or privileges.
“Imminent Radiation Hazard(s)” means an imminent hazard exists when the radiation levels that exist are in excess of three times the regulatory limit.
“License” means a license issued by the Agency in accordance with the regulations.
“Licensee” means any person who is licensed by the Agency in accordance with the regulations and the Act.
"Licensed Practitioner" means an individual licensed to practice medicine, dentistry, dental hygiene, podiatry, chiropractic, or osteopathy in this State.
“Licensee's Representative” means a person who has been authorized by the licensee to represent them during activities or proceedings governed by the regulations.
“Modification” means a change in the specification of a machine or radiation facility.
“Notice of violation” means a written statement of one or more alleged infringements of a legally binding requirement. The notice normally requires the licensee, registrant, other permit holder to provide a written statement describing the following:
corrective steps taken by the licensee, registrant, or other permit holder, and the results achieved;
corrective steps to be taken to prevent recurrence; and
the projected date for achieving full compliance. The Authority may require responses to notices of violation to be under oath.
Registration means to enroll or register with the Agency in accordance with the regulations.
Regulations mean all parts of the Delaware Radiation Control Regulations (DRCR) and all parts of the Delaware Radiation Technologist Certification Regulations (RTCR).
Severity level means a classification of violations based on relative seriousness of each violation and the significance of the effect of the violation on the occupational or public health or safety.
Violation means an infringement of any rule, certificate, license or registration condition, order of the agency, or any provision of the Act.
3.1 Certificate
3.1.1 A Radiation Technologist Certificate is required to practice radiation technology (Request Application Form ORC-R16) as outlined in the Delaware Radiation Technologist Certification Regulations, Regulation 4466.
3.1.2 A Plan Approval Certificate is required for the construction or modification of a radiation facility (Request Shielding Information Letter) as outlined in the regulations.
3.2 License
3.2.1 A Radioactive Material License is required to use and/or possess a radioactive material source (Request Application Form ORC-R2).
3.3 Registration
3.3.1 A Registration is required to possess and/or use a radiation machine (i.e. x-ray equipment) or operate a radiation machine facility. (Request Application Form ORC-R1)
3.3.2 Registration is required to possess a source of Radioactive Material. (Request Application Form ORC-R2)
3.3.3 A Registration is required to perform a radiation service including but not limited to, repair, install or calibrate radiation equipment/devices, perform health physics or radiation protection consultations or surveys, personnel dosimetry and therapeutic radiation physics (Request Application Form ORC-R3).
3.4 Annual/Biennial License or Registration
3.4.1 An annual or biennial license or registration shall be issued to any person desiring to possess, use, provide or operate a source of radiation, radiation facility or radiation service in the State for more than thirty (30) days upon written application to the Agency.
3.4.2 The Agency shall issue a license or registration to the applicant if the Agency's inspection or examination reveals that the proposed facility, use, source of radiation and/or individual complies with the requirements of the regulations.
3.4.3 An annual/biennial license or registration is valid for one (1) or two (2) anniversary year(s) from the date of issuance, unless a new owner, management, firm or lessee takes possession of the facility, source of radiation or service; or the license or registration is revoked by the Authority for violations of the regulations. A license or registration is not transferable.
3.5 Temporary Use of Out of State Source
3.5.1 This license or registration allows for the temporary use of an out-of-state radiation source in Delaware.
3.5.2 X-ray equipment - send advance written notice to the Agency as per Regulation 4481 (Part B).
3.5.3 Radioactive Material Source/Device - send advance written notice to the Agency of the intended use and location with photo copy of NRC or Agreement State License as per Part C.
4.1 A valid certificate, license or registration is not transferable. Therefore, it is the responsibility of the new owner/manager to acquire an operating certificate, license or registration prior to commencing operations.
4.2 New construction or modification of an existing room or area associated with a source of radiation requires plan approval in accordance with Regulation 4481.
4.3 The owner/manager of a radiation source is responsible for notifying the Agency prior to its sale, transfer, lease, or disposal in accordance with requirements listed in Regulations 4481 (Part B).
4.4 If any renovations or modifications of the physical structure of the existing facility are required, based on current or previous inspection reports of the Agency, the new owner/manager will be held responsible for these renovations or modifications.
4.4.1 Completion of any renovations shall be achieved prior to the start of operation, unless the new owner/manager is granted an exemption in accordance with Regulation 4480.
5.1 Inspection Frequency.
5.1.1 An inspection of a registered facility shall be performed at least every two (2) years for medical facilities utilizing angiography, radiography, fluoroscopy, computed tomography (CT), mammography, stereotactic breast biopsy systems and radiation therapy modalities, and at least every four (4) years for other registered facilities, including dental, bone densitometry, podiatry, veterinary, academic and industrial.
5.1.2 Additional inspections of registered facilities as described in the regulations shall be performed as often as necessary to ensure and verify compliance with the regulations.
5.2 Access
5.2.1 The Authority or its duly authorized representatives shall have the power to enter at all reasonable times upon any private or public property for the purpose of determining whether or not there is compliance with or violations of 16 Del.C., Ch.74 and rules and regulations issued thereunder, except that entry into areas under the jurisdiction of the federal government shall be effected only with the concurrence of the federal government or its duly authorized designated representative.
5.2.2 The Agency may suspend for a period not to exceed (30) days, the certificate, license or registration to operate/use a source of radiation for refusing access to the representative(s) of the Agency if the Agency can show good cause that there is a risk of imminent harm to the public from ionizing radiation at the facility to which the Agency is attempting access.
5.3 Inspection Report Form
5.3.1 Facility Inspection Form ORC-R11 shall be used to record the results of inspections at registered radiation source facilities as specified in (Part K), Compliance Procedures.
5.3.1.1 The co-signed original of the completed inspection report form shall be furnished to the person named on the license or registration or the Radiation Safety Officer (RSO) at the conclusion of the inspection.
5.3.1.2 The completed inspection form shall list the violation(s) (if any), give the time period for correcting the violation(s) and state the corrections to be made. The inspection report form shall summarize the requirement(s) of the regulations.
5.3.1.3 The form shall also state that "Failure to comply with time limits for correction of any violations cited in this notice shall result in automatic license or registration suspension and immediate cessation of use of a source radiation or radiation area in accordance with Compliance Procedures of the regulations of the Authority on Radiation Protection."
5.3.2 The completed inspection report form is a public document that shall be made available for public disclosure to any person who requests it in accordance with the "Freedom of Information Act 29 Del.C., Ch. 100."
5.3.3 A Notice of Violation (Form ORC-R10) must be posted in a conspicuous location until the violation has been corrected.
5.4 Types of Inspections. Inspections are performed to verify compliance with all applicable laws and regulations.
5.4.1 Regular Inspections
5.4.1.1 Regular inspections are performed on a routine basis in permanent, operating, registered facilities. These inspections shall address all items on the inspection report form. Items in violation shall be recorded by item number.
5.4.2 Follow-up Inspections
5.4.2.1 Follow-up inspections shall be performed when a regular inspection finds one (1) or more Severity Level 1 violation(s) or three (3) or more Severity Level 2 violations.
5.4.2.2 Follow-up inspections may also be performed to verify proper posting of certificates, licenses or registrations, after complaint and investigation inspections, or after administrative hearings.
5.4.3 Complaint Inspections
5.4.3.1 Complaint inspections are performed in response to formal or informal complaints against registered facilities.
5.4.3.2 A complete inspection may be performed by the Agency in the interest of protecting the public.
5.4.4 Investigation Inspections
5.4.4.1 Investigation inspections are performed on non-registered radiation facilities for determining whether compliance with the regulations is required.
5.4.5 Other Inspections
5.4.5.1 Other inspections include construction/modification, pre-operational and other inspections not included above.
6.1 Violations of the regulations have been classified as Severity Level 1 and Severity Level 2 depending upon the impact of the violation.
6.1.1 Severity Level 1 violations generally could result in overexposure to the patient or operator or violate individual's rights as outlined in the regulations.
6.1.2 Severity Level 2 violations generally will not result in overexposure but may indicate a lack of administrative controls over the use of the radiation source. Reference Appendix A for violation classifications.
6.2 Severity Level 1 Items
6.2.1 When one (1) or two (2) Severity Level 1 items of violation are found by any inspection, the related source(s) of radiation shall be tagged "out-of-use." All violations shall be corrected prior to returning the unit in service.
6.2.2 When three (3) or more Severity Level 1 items of violation are found by any inspection, the certificate, license or registration shall be suspended in accordance with Compliance Procedures. All violations shall be corrected prior to resuming registered activities.
6.2.3 The licensee or registrant shall inform the Agency in writing within 10 days of issuance of the inspection report of the proposed method or means of correcting the Severity Level 1 violation(s) and of the date when the correction will be made.
6.2.4 Follow-up inspections shall be conducted within 30 days to assure correction.
6.3 Severity Level 2 Items
6.3.1 All Severity Level 2 items shall be corrected as soon as possible, but in any event, within 60 days.
6.3.2 Follow-up inspections shall be conducted within 60 days to assure corrections have been completed.
6.4 If a follow-up inspection of a registered facility indicates non-compliance of a previously cited violation of the last inspection, a hearing before the Authority on Radiation Protection shall be scheduled. Additionally, the Agency may file a complaint to the Authority.
7.1 Radiation Machine Facility Permit Fees are established for issuance of annual registration permits to radiation machine facilities located within the State of Delaware, in accordance with 16 Del.C., Ch 74.
7.2 Fee Schedule
Category I: Facilities with a total of five or more of the medical modalities or non-medical modalities listed below: $1370.
Category II: Facilities with a total of three or four of the medical modalities or non-medical modalities listed below: $1030.
Category III: Facilities with two of the medical modalities listed below: $690.
Category IV: Facilities with one of the medical modalities listed below, and an annual patient workload of 750 examinations or more: $275.
Category V: Facilities with one of the medical modalities listed below, and an annual patient workload of less than 750 examinations, and all other radiation installations with one or two of the non-medical modalities listed below except as listed under Category VI: $140.
Category VI: Dental, podiatric, bone densitometry or veterinary installations: $75.
7.3 Fee Category Definitions
7.3.1 For purposes of the fee schedule set out in 7.2 above, the following definitions shall apply:
“medical modalities” shall mean radiography, fluoroscopy, computed tomography, angiography, stereotactic breast biopsy systems, and radiation therapy, utilized on humans.
7.3.2 For purposes of the fee schedule set out in 7.2 above, the following definitions shall apply: “non-medical modalities” shall mean radiography, fluoroscopy, analytical equipment (including electron microscopes, fluorescence analysis and x-ray diffraction equipment), computed tomography, and particle accelerators, not utilized on humans.
8.1 If the Agency determines that condition(s) exist(s) in a registered facility which represent(s) a threat to life or a serious risk of damage to health, safety and welfare of the workers or public, or if serious violations, repeat violations, or general disregard of accepted radiation practice are found to exist, administrative action is required.
8.2 Compliance Conference. A meeting held by the Agency with management of a licensee, registrant, or other license, certificate or registration holder to discuss the following:
8.2.1 safety, safeguards, or environmental problems;
8.2.2 compliance with regulatory, license condition, or registration condition requirements;
8.2.3 proposed corrective measures including, but not limited to, schedules for implementation; and
8.2.4 enforcement options available to the agency.
8.3 Suspension of Certificate, License or Registration
8.3.1 Conditions for Suspension of Certificate, License or Registration
8.3.1.1 If some condition(s) is/are determined to exist in a registered facility which present(s) an imminent radiation hazard to human health, the Agency may cease operations of the source of radiation without a hearing or written notice until such time the conditions have been corrected.
8.3.1.2 Further enforcement action shall be taken in accordance with the regulations.
8.3.1.3 Such an imminent radiation hazard shall include, but is not limited to, any one of the following:
8.3.2 The suspension shall be effective upon receipt of written notice by the Radiation Safety Officer or the person in charge of the radiation facility or their agent.
8.3.2.1 A suspension statement recorded on the inspection report by the Agency constitutes a written notice.
8.3.2.2 Service of a written notice of suspension by the Agency stating the reason(s) for the suspension must be made by the close of the following business day.
8.3.2.3 The certificate, license or registration shall not be suspended for a period longer than necessary to correct the hazardous conditions, unless mutually agreed upon.
8.4 Right of Appeal of Suspension
8.4.1 The owner/manager of the registered facility may submit in writing an appeal to the Authority on Radiation Protection for reconsideration of a decision by the Agency.
8.4.2 The notice of appeal shall be sent via certified mail to the Authority on Radiation Protection and to the Agency. An appeal shall not automatically stay the decision of the Agency.
8.4.3 After review for potential radiation hazard to the public, the suspended or revoked license or registration may be stayed on the order of the Program Administrator for Radiation Control.
8.4.4 If a notice of appeal is not filed within thirty (30) days, the license or registration suspension or revocation recommendation shall be upheld and other enforcement action taken in accordance with the this regulation, section 9.4. If the notice of appeal is timely filed, the Authority on Radiation Protection shall hold a hearing at its earliest opportunity.
8.5 Reinstatement of Certificate, License or Registration:
8.5.1 In consultation with the Authority on Radiation Protection, if a follow-up inspection by a representative of the Agency shows the imminent radiation hazard(s) to human health no longer exist(s), the suspension shall be lifted immediately and the certificate, license or registration returned.
8.5.2 If there is no evidence that the imminent radiation hazard(s) has/have been corrected, the suspension will remain in effect until the condition(s) has been corrected.
8.5.3 The owner/manager of the registered facility may request, in writing, a hearing before the Authority on Radiation Protection at any time during the period of suspension, for the purpose of demonstrating that the imminent radiation hazard(s) no longer exist.
8.5.4 The request for hearing shall not stay the suspension.
8.5.5 A record of all proceedings shall be made in accordance with Compliance Procedures.
8.6 Exemption Requests
8.6.1 All requests for exemptions must be filed with the Agency for review. If a determination cannot be made by the Agency, the exemption request must be referred to the Authority.
8.6.2 Agency may grant, an exemption if based on national standards.
8.6.3 Shall hear an appeal by the applicant within ten (10) days following the denial of an exemption request by the Agency. If an exemption is granted; record of the action shall become a part of the permanent record of the facility.
8.6.4 An exemption is not transferable.
9.1 Administrative Hearings
9.1.1 The Authority may, upon sworn complaint or upon its own initiative, cause an investigation to be held to determine whether a license or registration holder, former license or registration holder or applicant has engaged in any activity requiring disciplinary action.
9.1.2 Upon completion of said investigation, the Authority shall hold a hearing to determine whether a license or registration holder, former license or registration holder or applicant has engaged in activities specified in this section as grounds for disciplinary action.
9.1.3 The Authority shall fix the time and place for the hearing.
9.2 The Authority shall cause a copy of the charges, together with a notice of the time and place for the hearing, to be served on the alleged violator at least 30 days prior to the date fixed for the hearing.
9.2.1 When personal service cannot be effected, the Agency shall mail a copy of the charges and of such notice to the alleged violator at his last known address according to the records of the Agency.
9.3 In all proceedings herein:
9.3.1 The alleged violator may be represented by counsel who shall have the right of examination and cross-examination.
9.3.2 The alleged violator and the Agency may call witnesses and admit documentary evidence on their own behalf.
9.3.3 Testimony before the Authority shall be under oath. Any member of the Authority shall have power to administer oaths for this purpose.
9.3.4 A record of the hearing shall be made.
9.3.4.1 At the request and expense of either party such record shall be transcribed with a copy to the other party.
9.3.5 The decision of the Authority shall be based upon a preponderance of the evidence.
9.3.5.1 If the charges are supported by such evidence, the Authority may revoke, refuse to issue, or suspend a certificate, license or registration, or otherwise discipline the individual.
9.3.5.2 A suspended certificate, license or registration may be reissued upon a further hearing initiated at the request of the suspended licensee by written application in accordance with the rules of the Authority and if the Authority finds compliance has been achieved.
9.4 Revocation and Appeal of Suspended Certificate, License or Registration
9.4.1 The Authority on Radiation Protection, at its earliest opportunity, shall consider the Agency's recommendation for certificate, license or registration revocation or hear an appeal by the owner/manager whose permit stands suspended.
9.4.2 The Authority on Radiation Protection shall, at each scheduled meeting, release the name(s) and address(es) of those registered facilities currently meeting the following criteria:
9.4.3 Certificate, License or Registration permanently revoked
9.4.4 Certificate, License or Registration suspended
9.4.5 Certificate, License or Registration censored
9.4.6 Issued a letter of reprimand
9.4.7 Certificate, License or Registration application refused
9.4.8 Certificate, License or Registration renewal refused
9.4.9 Use of source of radiation terminated
9.5 Exemptions referred to the Authority
9.5.1 The Authority on Radiation Protection:
9.5.2 May from time to time grant written permission to vary from particular provisions set forth in the regulations when the extent of the variation is clearly specified and it is documented to the Authority's satisfaction that:
9.5.2.1 Such variation is necessary to obtain a beneficial use by the owner/manager of an existing facility;
9.5.2.2 Appropriate alternative measures have been taken to protect the health and safety of the public from ionizing radiation and assure that the purpose of the provisions from which the variation is sought will be observed.
9.5.3 The Authority shall hear an appeal by the applicant within thirty (30) days following the denial of an exemption request by the Agency. If an exemption is granted; record of the action shall become a part of the permanent record of the facility.
9.5.4 An exemption is not transferable.
10.1 Inspection/Enforcement
10.1.1 A registered facility may be inspected by the Agency as often as necessary for enforcement of the regulations.
10.1.2 Registered facilities, their employees and their agents shall be in compliance with the regulations.
10.1.3 The established administrative procedures for the implementation and enforcement of the provisions and penalties of 16 Del.C., Ch, 74, shall be applicable to this section.
10.2 Failure to allow access, inspection or tests by the Agency representative(s) shall cause the Agency to prohibit the use of a source of radiation, close the registered facility, and/or suspend the facility certificate, license or registration if the Agency can show good cause to believe that there is a risk of immediate harm to the public from ionizing radiation at the facility to which the Agency is attempting access.
10.3 Procedure when Overexposure/Radiation Contamination is Suspected:
10.3.1 When the Agency has reasonable cause to suspect possible individual overexposure or radioactive contamination at a registered facility in excess of limits set forth in regulation 4483 (Part D) it may conduct a radiation investigation which can indicate exposure histories of individuals or make any other investigations as indicated and shall take appropriate action.
10.3.2 The Agency may require any or all of the following measures:
10.3.2.1 The immediate closing of the registered facility or prohibition of the use of a radiation source or radiation area until, in the opinion of the Agency, no further danger of overexposure or contamination exists.
10.3.2.2 Restriction of an employee(s) services to some area of the radiation facility where there would be no opportunity to use a source of radiation or be irradiated.
10.3.2.3 Any other action which the Agency can demonstrate is necessary to protect the health of the public and other employees of the radiation facility.
11.1 Any person who violates a provision of the regulations and any person who is the holder of a certificate, license or registration or who otherwise operates a registered facility that does not comply with the requirements of the regulations shall be subject to the provisions of 16 Del.C. section 7416.
11.2 Operation without a Certificate, License or Registration:
11.2.1 If a facility or individual is found operating without a valid certificate, license or registration as required by Regulation 4481 (Part B) or Regulation 4466, section 7.0 (previously referenced as DRCR B.5. or RTCR, section 7.0 of the regulations), the Agency may act on behalf of the Authority and the source of radiation shall be tagged out-of-use.
11.3 The Agency may seek to enjoin violations of the regulations.
11.4 A conspicuous notice shall be prominently displayed on the radiation source or at all entrances of facilities meeting the following criteria:
11.4.1 Failed to obtain a valid certificate, license or registration; or
11.4.2 Certificate, License or Registration suspended; or
11.4.3 Certificate, License or Registration revoked.
APPENDIX A
Violation Classification (Typical/Not all inclusive)
Radiation Source Facility
Severity Level 1 | Severity Level 2 | ||
1. | Operating without a permit, (PART K), section 1.2 1.1 | 1. | Registration Form 16. Del.C. 7402, (PART K), section 1.0 |
2. | Personnel Overexposure, Regulation 4483, section 6.0 (D. 201) | 2. | Notice to Employees, Regulation 4489, section 2.3 (J.11) |
3. | Radiation Levels Excessive, Regulation 4483, sections 20.0 and 21.0 (D.601,D.602,D.603) | 3. | Safety Procedures, (PART F), section 3.1 (D.101,D.1102) |
4. | Personnel Monitoring, Regulation 4483, section 18.0 (D.502) | 4. | Personnel Monitoring Records, Regulation 4483, section 46.0 (D.1107) |
Medical Uses
Severity Level 1 | Severity Level 2 | ||
1. | Patient exposure (ESE) not in accordance with (PART F), section 3.1.4 (F.3.a.iv) | 12. | |
2. | Tube support not in accordance with (PART F), section 4.7 (F.4.g) | 13. | Maintenance of x-ray system records not in accordance with (PART F), section 3.1.7 (F.3.xiv) |
3. | Technique indicators not in accordance with (PART F), section 4.8 (F.4.h) | 14. | Speed of film/screen not in accordance with (PART F), section 3.1.9 (F.3.ix.1) |
4. | Multiple tube indication not in accordance with (PART F), section 4.6 (F.4.f) | 15. | Technique chart not in accordance with (PART F), section 3.1.3 (F.3.a.iii) |
5. | Gonadal shielding not in accordance with (PART F), section 3.1.6 (F.3.a.vi) | 16. | Warning label not in accordance with (PART F), section 4.1 (F.4.a) |
6. | Operator apron/barrier not in accordance with (PART F), section 3.1.5.2 (F.3.viii.6) | 17. | Patient dose of 1 gray (100 rads) or more not reported to the Agency, (PART F), section 5.11 (F.5.k) |
Radiographic
Severity Level 1 | Severity Level 2 | ||
1. | Location of x-ray controls not in accordance with (PART F), sections 6.25; 7.35 (F.6.b.v and F.7.c.v.1) | 1. | Visual/audio signal not in accordance with (PART F), sections 6.22; 7.32 (F.6.b.ii and F.7.c.ii) |
2. | Position indicating device not in accordance with Regulation 4480, section 6.3 (F.4.j) | 2. | Adjustment of x-ray field not in accordance with (PART F), section 6.11 (F.5.iii) |
3. | Beam Collimation not in accordance with (PART F), section 7.2 (F.6.h.i) | 3. | Indication of field size, upon adjustments, not in accordance with (PART F), section 6.1.2 (F.6.a.ii2) |
4. | Filtration deficiency of 0.2+ mm not in accordance with (PART F), section 4.5.1 (F.4.e.ii) | 4. | Means to limit source to skin distance not in accordance with (PART F), section 7.1 (F.7.a) |
5. | Variation in timer linearity of 15% or more not in accordance with (PART F), sections 6.2.4; 7.3.4 (F.6.b.iv and F.6.g) | 5. | Filtration deficiency of 0.2 mm or less not in accordance with (PART F), section 4.5.1 (F.4.e.ii) |
6. | Variation in exposure reproducibility of 15% or more not in accordance with (PART F), sections 6.4; 7.4 (F.6.d and F.7.d) | 6. | Variation in timer reproducibility linearity more that 10% but less than 15% not in accordance with (PART F), section 6.2.4 (F.6.b.iv) |
7. | Total misalignment of x-ray/light field edges of 5% or more not in accordance with (PART F), section 6.1.2.1 (F.6.i.2) | 7. | Variation in exposure reproducibility more than 5% but less than 10% not in accordance with (PART F), sections 6.4; 7.4 (F.7.d and F.6.vii.d) |
8. | Total misalignment of x-ray beam/image receptor centers of 5% or more not in accordance with (PART F), section 6.1.2.1 (F.6.i.2) | 8. | Total misalignment of light/x-ray field edges more than 2% but less than 5% not in accordance with (PART F), section 6.1.1.2 (F.6.a.iv.i) |
9. | Discorrespondence of indicated x-ray field with beam limited x-ray field of 5% or more not in accordance with (PART F), section 6.1.2.3. (F.6.a) | 9. | Total misalignment of x-ray beam/image receptor centers more than 2% but less than 5% not in accordance with (PART F), section 6.1.2.1 (F.6.a.iv.2) |
10. | Discorrespondence of indicated x-ray with beam limited x-ray field more than 2% but less than 5% not in accordance with (PART F), section 6.2.3 (F.6.a.i.2) | ||
Fluoroscopic
Severity Level 1 | Severity Level 2 | ||
1. | Activation of x-ray production not in accordance with (PART F), section 5.4. (F.5.b) | 1. | Posting of exposure rate measurements not in accordance with (PART F), section 5.3 (F.5) |
2. | Annual exposure rate measurement not in accordance with (PART F), section 5.3.1.4 (F.5.4) | 2. | Measurement records and posting of same not in accordance with (PART F), section 5.3 (F.5) |
3. | Exposure rate not in accordance with (PART F), section 5.3 (F.5.c) | 3. | Adjustment of field not in accordance with (PART F), section 5.1.2 (F.5.a.ii) |
4. | Useful beam protective barrier not in accordance with (PART F), section 5.4 (F.5.d) | 4. | Means of field adjustment not in accordance with (PART F), section 5.1.2 (F.5.a.ii) |
5. | Limitation of x-ray field to image receptor not in accordance with (PART F), section 5.1 (F.5) | 5. | Indication of kV and mA not in accordance with (PART F), section 5.5 (F.5.4) |
6. | Minimum field at maximum SID not in accordance with (PART F), section 5.1.2.2.2 (F.5.iii3) | 6. | Means to indicate that beam is perpendicular to image receptor not in accordance with (PART F), section 5.1 (F.5.2.d) |
7. | High level control exposure rate limit not in accordance with (PART F), section 5.3 (F.5.c) | 7. | Audible signal to reset not in accordance with (PART F), section 5.7 (F.5.c.b and F.5.g) |
8. | Timing device not in accordance with (PART F), section 5.7 (F.5.g) | 8. | Minimum source to skin distance not in accordance with (PART F), section 5.6 (F.5.f) |
9. | Control of scattered radiation not in accordance with (PART F), section 5.8 (F.5.h) | 9. | Exposure rate due to transmission through primary barrier not in accordance with (PART F), section 5.4 (F.5.a.1) |
10. | Failure to report patient dose of 1+ gray to the Agency, (PART F), section 5.11 (F.5.k) | ||
Industrial Radiographic
Severity Level 1 | Severity Level 2 | ||
1. > 2 mR/hr. | Failure to maintain radiation level from device < 4" in diameter below maximum allowable limit of 50 mR/hr. at 6" in accordance with Regulation 4484, section 5.1 (E.101) | < 2 mR/hr. | |
2. > 2 mR/hr. | Failure to maintain radiation level from device > 4" diameter below maximum allowable limit of 200 mR/hr. at surface in accordance with Regulation 4484, section 5.1 (E.101) | < 2 mR/hr. | |
3. > 2 mR/hr. | Failure to maintain radiation level from storage container below maximum allowable limit of 200 mR/hr. at the surface in accordance with Regulation 4484, section 5.1 (E.101) | < 2 mR/hr. | |
4. > 2 mR/hr. | Failure to maintain radiation level from storage container below maximum allowable limit of 10 mR/hr. at a distance of 1 meter in accordance with Regulation 4484, section 5.1 (E.101) | < 2 mR/hr. | |
Locking of Source | |||
5. | Radiation Source Storage Container was found unlocked and not under the direct surveillance of authorized individual in accordance with Regulation 4484, section 5.2.1 (E.102) | ||
6. | Exposure device and/or storage container was not locked during transit or prior to unit being made secure as required by Regulation 4484, section 5.2.2 (E.103) | ||
7. | Device was not physically secured to prevent tampering or removal as required by Regulation 4484, section 5.3 (E.103) | ||
Survey Instruments
Severity Level 1 | Severity Level 2 | ||
1. | Failure to adequately maintain survey instruments in accordance with Regulation 4484, section 5.4.1 (E.104) | 1. | Failure to possess calibrated back up instrument Regulation 4484, section 5.4.1 (E.104) |
2. | Survey meter not capable of detecting 2-1000 mR/hr. as required by Regulation 4484, section 5.4.1 (E.104) | 2. | Failure to conduct quarterly calibrations in accordance with Regulation 4484, section 5.4.2 (E.106) |
3. | Failure to calibrate unit after servicing in accordance with Regulation 4484, section 5.4.2 (1st bullet point) (E.104) | ||
4. | Failure to achieve accuracy of (+ 20%) as required by Regulation 4484, section 5.4.2 (2nd bullet point) (E.104) | ||
5. | Failure to calibrate each scale using 2 points other than zero as required by Regulation 4484, section 5.4.2 (3rd bullet point) (E.104) | ||
6. | Failure to maintain calibration records for two years in accordance with Regulation 4484, section 5.4.3 (E.107) | ||
Leak Testing/Repair
Severity Level 1 | Severity Level 2 | ||
1. | Service to sealed source not conducted by authorized individual in accordance with Regulation 4484, section 5.5.1 (E.105) | 1. | Failure to conduct leak test within 6 month interval as required by Regulation 4484, section 5.5.2 (E.105) |
2. | Failure to immediately withdraw contaminated unit from service to effect decontamination/repair as required by Regulation 4484, section 5.5.4 (E.105) | 2. | Leak test procedure not capable of detecting unit 0.005 uCi of removable contamination as required by Regulation 4484, section 5.5.2 (E.105) |
3. | Failure to properly label unsecured source in accordance with Regulation 4484, section 5.5.5 (E.105) | 3. | Failure to record test results in microcuries as required by Regulation 4484, section 5.5.2 (E.105) |
4. | Failure to notify agency within 5 days of receiving test results as required by Regulation 4484, section 5.5.4 (E.105) | ||
Inventory/Inspection
Severity Level 1 | Severity Level 2 | ||
1. | Failure to perform quarterly operational safety maintenance and inspection in accordance with Regulation 4484, section 5.8.2 (E.108) | 1. | Failure to conduct quarterly inventory as required by Regulation 4484, section 5.6 (E.106) |
2. | Failure to withdraw defective unit from service as required by Regulation 4484, section 5.8.3 (E.109) | 2. | Failure to maintain utilization log as required by Regulation 4484, section 5.7 (E.107) |
3. | Failure to conduct and record alarm tests at the beginning of each period of use as required by Regulation 4484, section 5.9.2 (E.109, E.202) | 3. | Failure to log required information in accordance with Regulation 4484, sections 5.7.1 - 5.7.4 (E.107) |
4. | Failure to properly document quarterly inspection and maintenance of radiation devices in accordance with Regulation 4484, section 5.8 (E.108) | ||
5. | Failure to record and maintain test results in accordance with Regulation 4484, section 5.9 (E.303) | ||
Limitations/Procedures/Controls
Severity Level 1 | Severity Level 2 | ||
1. | Individual is not qualified Industrial Radiographer as defined by Regulation 4484, sections 10.1.1.1 - 10.1.1.3 (E.201) | 1. | Failure to maintain training and test records in accordance with Regulation 4484, section 10.1.3 (E.201) |
2. | Individual is not a qualified radiographer's assistant trainee as defined by Regulation 4484, section 10.1.2 (E.201) | 2. | Failure to record dosimetry data in accordance with Regulation 4484, section 10.3.2 (E.201) |
3. | Qualified operator(s) not wearing personal monitoring devices as required by Regulation 4484, section 10.3.1 (E.203) | ||
4. | Operating and Emergency procedures do not meet the requirements of Regulation 4484, sections 10.2.1.1 - 10.2.1.7 (E.203) | ||
Precautionary Procedures
Severity Level 1 | Severity Level 2 | ||
1. | Failure of qualified operators to maintain direct surveillance in accordance with Regulation 4484, section 11.1 (E.301) | 1. | Failure to post required information in accordance with Regulation 4484, section 11.2 (E.302) |
2. | Failure to have calibrated and operable survey instrument available in accordance with Regulation 4484, section 11.3.1 (E.303) | 2. | Failure to maintain survey records for 2 years in accordance with Regulation 4484, section 11.3.6 (E.303) |
3. | Failure to conduct physical radiation survey in accordance with Regulation 4484, section 11.3.2 and/or 11.3.3 (E.303) | ||
4. | Failure to maintain records at temporary job site in accordance with Regulation 4484, sections 11.4.1 - 11.4.6 (E.304) | ||
Special Requirements
Severity Level 1 | Severity Level 2 | ||
1. | Failure to meet requirements for enclosed radiography in accordance with Regulation 4484, section 11.5.1 (E.306) | 1. | Failure to maintain evaluation records for 2 years in accordance with Regulation 4484, section 9.1.2 (E.306) |
2. | Failure to meet exemption requirements and maintain unit in accordance with Regulation 4484, section 11.5.2 (E.306) | ||
Analytical
Severity Level 1 | Severity Level 2 | ||
1. | Failure to provide safety device in accordance with Regulation 4487, section 3.1 (H.3) | 1. | Failure to label equipment in accordance with Regulation 4487, section 3.4 (H.3) |
2. | Failure to provide warning devices in accordance with Regulation 4487, section 3.2 (H.3) | 2. | Failure to have appropriate warning lights in accordance with Regulation 4487, section 3.2 (H.3) |
3. | Failure to install shutter in accordance with Regulation 4487, section 3.5 (H.3) | 3. | Failure to provide protective cabinet to prevent leakage in accordance with Regulation 4487, section 3.7 (H.3) |
4. | Failure to construct tube housing in manner which ensures compliance with Regulation 4487, section 3.6 (H.3) | 4. | Failure to conduct Radiation Surveys in accordance with Regulation 4487, section 4.2 (H.4) |
5. | Failure to obtain Radiation Safety Officer's approval for by-passing safety device in accordance with Regulation 4487, sections 5.1 and 5.2 (H.5) | 5. | Failure to document survey record in accordance with Regulation 4487, section 4.2 (H.4) |
6. | Failure to provide and/or ensure the use of extremity dosimeters in accordance with Regulation 4487, section 6.2 (H.6) | 6. | Failure to post area in accordance with Regulation 4487, section 4.3 (H.4) |
7. | Failure to instruct and/or ensure operator competence in accordance with Regulation 4487, section 6.1 (H.6) | 7. | Failure to secure unused ports in accordance with Regulation 4487, section 3.3 (H.3) |
Particle Accelerators
Limitations
Severity Level 1 | Severity Level 2 | ||
1. | Operator did not receive radiation safety instruction or could not demonstrate understanding of radiation safety Regulation 4488, section 6.1 (I.6) | 1. | Operator had not received copies Regulations 4483, 4488, 4489 & emergency procedures, Regulation 4488, section 6.1 (I.6) |
2. | Operator unable to demonstrate competency in the use of the accelerator Regulation 4488, section 6.1 (I.6) | 2. | Radiation Safety Committee/Radiation Safety Officer is not authorized to terminate operations as per Regulation 4488, section 6.2 (I.6) |
Controls/Interlocks
Severity Level 1 | Severity Level 2 | ||
1. | HRA not provided with Interlock as per Regulation 4488, section 8.2 (I.8) | 1. | Instrumentation and controls are not clearly identified Regulation 4488, section 8.1 (I.8) |
2. | Interlock does not require manual reset Regulation 4488, section 8.5 (I.8) | 2. | Scram buttons do not require manual reset Regulation 4488, section 8.6 (I.8) |
3. | Safety interlocks not independently wired Regulation 4488, sections 8.3 and 8.4 (I.8) | 3. | Not all HRA entrances are equipped with warning lights as per Regulation 4488, section 9.1 (I.9) |
4. | All safety interlocks are not fail safe Regulation 4488, section 8.4 (I.8) | 4. | Not all HRA are equipped with audible warning devices which activate for 15 seconds Regulation 4488, section 9.2 (I.9) |
5. | Scram buttons not located in HRA Regulation 4488, section 8.6 (I.8) | 5. | HRA barriers/pathways are not identified per Regulation 4483, section 8.0; Regulation 4488, section 9.3 (D.801, I.9) |
Operating Procedures
Severity Level 1 | Severity Level 2 | ||
1. | Particle accelerator not unsecured from unauthorized use Regulation 4488, section 10.1 (I.10) | ||
2. | Bypass of safety interlock not authorized by Radiation Safety Officer and/or Radiation Safety Committee or not recorded and posted as per Regulation 4488, section 10.5 (I.10) | 2. | Safety interlocks are used as routine "off" switch Regulation 4488, section 10.2 (I.10) |
3. | Electrical circuit diagram not maintained and available Regulation 4488, section 10.4 (I.10) | ||
4. | Current operating and emergency procedures are not kept at control panel Regulation 4488, section 10.6 (I.10) | ||
5. | Record of quarterly safety device operability check are not available Regulation 4488, section 10.3 (I.10) | ||
Radiation Monitoring
Severity Level 1 | Severity Level 2 | ||
1. | Radiation protection survey not performed and/or documented by an approved person following operation or facility changes Regulation 4488, section 11.2 (I.11) | 1. | Portable monitoring equipment not available, operable and calibrated Regulation 4488, section 11.1 (I.11) |
2. | Radiation levels not continuously monitored in all HRAs as per Regulation 4488, section 11.3 (I.11) | 2. | All area monitors are not calibrated annually Regulation 4488, section 11.4 (I.11) |
3. | Periodic surveys of airborne particulates are not performed as per Regulation 4488, section 11.5 (I.11) | ||
4. | Periodic Smear Surveys are not conducted for contamination as per Regulation 4488, section 11.6 (I.11) | ||
5. | All area surveys are not performed according to proper written procedures as per Regulation 4488, section 11.7 (I.11) | ||
6. | Current records of all surveys and tests were not available at facility Regulation 4488, section 11.8 (I.11) | ||
Ventilation Systems
Severity Level 1 | |||
1. | Means are not provided to ensure compliance with Part D airborne concentrations Regulation 4488, section 12.1 (I.12) | ||
2. | Airborne concentrations in excess of Part D limits were discharged to an uncontrolled area Regulation 4488, section 12.2 (I.12) | ||
Notice and Reports to Workers
Posting of Notices
Severity Level 1 | Severity Level 2 | ||
1. | Regulations 4483 and 4489 of DRCR not posted, Regulation 4489, section 2.1 (J.11) | ||
2. | Facility radiation permit/document not posted Regulation 4489, section 2.1 (J.11) | ||
3. | Radiation operating procedures not posted Regulation 4489, sections 2.1 - 2.3 (J.11) | ||
4. | Notice of violation for radiological working conditions not posted Regulation 4489, section 2.1 (J.11) | ||
5. | Notice to employees, Agency Form X not posted Regulation 4489, section 2.3 (J.11) | ||
6. | Violations not posted within five (5) days nor remain posted for at least (5) days Regulation 4489, section 2.4 (J.11) | ||
7. | Documents do not appear in sufficient number of conspicuous places Regulation 4489, section 2.5 (J.11) | ||
Instructions to Workers
Severity Level 1 | Severity Level 2 | ||
1. | Workers were not instructed to report any conditions that could cause unnecessary exposure to radiation Regulation 4489, section 3.1 (J.12) | 1. | Workers were not kept informed of radiation sources Regulation 4489, section 3.1 (J.12) |
2. | Workers were not instructed about warnings for unusual occurrence or malfunction that may involve exposure to radiation Regulation 4489, section 3.1 (J.12) | 2. | Workers were not instructed of health protection problems associated with radiation exposure Regulation 4489, section 3.1. (J.12) |
3. | Workers were not advised of radiation exposure reports pursuant to Regulation 4489, sections 3.1.1 - 3.1.6; 4.0 (J.12) | 3. | Workers were not instructed to observe applicable parts of DRCR Regulation 4489, section 3.1 - 3.1.3 (J.12) |
Notifications
Severity Level 1 | Severity Level 2 | ||
1. | Written report of specified Radiation exposure data are not given to the worker Regulation 4489, section 4.1 (J.13) | 1. | Workers are not advised annually of their radiation exposure pursuant to Regulation 4489, section 3.1 (J.13) |
2. | Workers are not furnished a radiation exposure report within 30 days after the licensee becomes informed of exposure or termination of employment Regulation 4489, section 4.3 (J.13) | 2. | As required pursuant to D.405 D.1202, D.1203, or D.1204, exposed individuals are not provided a report of their radiation exposure as per Regulation 4489, section 4.4 (J.13) |
Representatives of Registrant/Workers
Severity Level 1 | |||
1. | Agency not afforded opportunity to inspect equipment and activities Regulation 4489, section 5.1 (J.14) | ||
2. | Agency not permitted to consult with workers privately Regulation 4489, section 5.2 (J.14) | ||
3. | Worker authorized representative not given opportunity to accompany agency during inspection of physical working conditions Regulation 4489, section 5.3 (J.14) | ||
4. | Worker representative does not meet qualifications set forth in routine radiation Regulation 4489, section 5.4 (J.14) | ||
5. | Different facility/worker representatives not permitted to accompany agency on inspection Regulation 4489, section 5.5 (J.14) | ||
6. | Mutually agreed upon "outside" individual(s) was not permitted to accompany agency inspectors Regulation 4489, section 5.6 (J.14) | ||
7. | Worker(s) was not allowed to privately consult with the Agency inspector about perceived radiological condition Regulation 4489, section 6.2 (J.15) | ||
8. | A worker has been discharged or discriminated against for filing a radiological complaint on behalf of himself or others Regulation 4489, section 6.3 (J.16) | ||
Therapeutic Radiation Machines
Severity Level 1 | Severity Level 2 | ||
1. | All non-compliance with the requirements of 4492 (Part X) are Severity Level 1 violations. | ||
Part X
1.1 This Part establishes requirements, for which the registrant is responsible, for use of therapeutic radiation machines. The provisions of this Part are in addition to, and not in substitution for, other applicable provisions of the regulations.
1.2 The use of therapeutic radiation machines shall be by, or under the supervision of, a licensed practitioner of the healing arts who meets the training/experience criteria established by Part X, Section 3, as applicable.
As used in this Regulation, the following definitions apply:
"Absorbed dose (D)" means the mean energy imparted by ionizing radiation to matter. Absorbed dose is determined as the quotient of dE by dM, where dE is the mean energy imparted by ionizing radiation to matter of mass dM. The SI unit of absorbed dose is joule per kilogram and the special name of the unit of absorbed dose is the gray (Gy). The previously used special unit of absorbed dose (rad) is being replaced by the gray.
"Conventional Simulator" means any x-ray system designed to reproduce the geometric conditions of the radiation therapy equipment.
"Detector" (See "Radiation detector").
"Dose monitor unit (DMU)" means a unit response from the beam monitoring system from which the absorbed dose can be calculated.
"Electronic brachytherapy" means a method of radiation therapy where an electrically generated source of ionizing radiation is placed in or near the tumor or target tissue to deliver therapeutic radiation dosage.
"Electronic brachytherapy device" means the system used to produce and deliver therapeutic radiation including the x-ray tube, the control mechanism, the cooling system, and the power source.
"Electronic brachytherapy source" means the x-ray tube component used in an electronic brachytherapy device.
"External beam radiation therapy" means therapeutic irradiation in which the source of radiation is at a distance from the body.
"Field‑flattening filter" means a filter used to homogenize the absorbed dose rate over the radiation field.
"Filter" means material placed in the useful beam to change beam quality in therapeutic radiation machines subject to 6.4.
"Gray (Gy)" means the SI unit of absorbed dose, kerma, and specific energy imparted equal to 1 joule per kilogram. The previous unit of absorbed dose (rad) is being replaced by the gray [1 Gy=100 rad].
"Half‑value layer (HVL)" means the thickness of a specified material which attenuates X‑radiation or gamma radiation to an extent such that the air kerma rate, exposure rate or absorbed dose rate is reduced to one‑half of the value measured without the material at the same point.
"Intensity Modulated Radiation Therapy (IMRT)" means radiation therapy that uses non-uniform radiation beam intensities which have been determined by various computer-based optimization techniques.
"Interlock" means a device preventing the start or continued operation of equipment unless certain predetermined conditions prevail.
"Interruption of irradiation" means the stopping of irradiation with the possibility of continuing irradiation without resetting of operating conditions at the control panel.
"Irradiation" means the exposure of a living being or matter to ionizing radiation.
"Isocenter" means the center of the sphere through which the useful beam axis passes while the gantry moves through its full range of motions.
"Kilovolt (kV) [kilo electron volt (keV)]" means the energy equal to that acquired by a particle with one electron charge in passing through a potential difference of one thousand volts in a vacuum. [Note: current convention is to use kV for photons and keV for electrons.]
"Lead equivalent" means the thickness of the material in question affording the same attenuation, under specified conditions, as lead.
"Leakage radiation" means radiation emanating from the radiation therapy system except for the useful beam.
"Licensed Practitioner" means an individual licensed to practice medicine, dentistry, dental hygiene, podiatry, chiropractic, or osteopathy in this State.
"Light field" means the area illuminated by light, simulating the radiation field.
"mA" means milliampere.
"Megavolt (MV) [mega electron volt (MeV)]" means the energy equal to that acquired by a particle with one electron charge in passing through a potential difference of one million volts in a vacuum. [Note: current convention is to use MV for photons and MeV for electrons.]
"Misadministration" means an event that meets the criteria in 5.2.
"Mobile Electronic Brachytherapy Service" means transportation of an electronic brachytherapy device to provide electronic brachytherapy at an address that is not the address of record.
"Monitor unit (MU)" (See "Dose monitor unit").
"Moving beam radiation therapy" means radiation therapy with any planned displacement of radiation field or patient relative to each other, or with any planned change of absorbed dose distribution. It includes arc, skip, conformal, intensity modulation and rotational therapy.
"Nominal treatment distance" means:
a. For electron irradiation, the distance from the scattering foil, virtual source, or exit window of the electron beam to the entrance surface of the irradiated object along the central axis of the useful beam.
b. For x‑ray irradiation, the virtual source or target to isocenter distance along the central axis of the useful beam. For non‑isocentric equipment, this distance shall be that specified by the manufacturer.
"Patient" means an individual subjected to machine produced radiation for the purposes of medical therapy.
"Peak tube potential" means the maximum value of the potential difference across the x‑ray tube during an exposure.
"Periodic quality assurance check" means a procedure which is performed to ensure that a previous parameter or condition continues to be valid.
"Phantom" means an object behaving in essentially the same manner as tissue, with respect to absorption or scattering of the ionizing radiation in question.
"Practical range of electrons" corresponds to classical electron range where the only remaining contribution to dose is from bremsstrahlung x‑rays. A further explanation may be found in "Clinical Electron Beam Dosimetry: Report of AAPM Radiation Therapy Committee Task Group 25" [Medical Physics 18(1): 73‑109, Jan/Feb. 1991] and ICRU Report 35, "Radiation Dosimetry: Electron Beams with Energies Between 1 and 50 MeV", International Commission on Radiation Units and Measurements, September 15, 1984.
"Prescribed dose" means the total dose and dose per fraction as documented in the written directive. The prescribed dose is a estimation from measured data from a specified therapeutic machine using assumptions that are clinically acceptable for that treatment technique and historically consistent with the clinical calculations previously used for patients treated with the same clinical technique.
"Primary dose monitoring system" means a system which will monitor the useful beam during irradiation and which will terminate irradiation when a pre‑selected number of dose monitor units have been delivered.
"Primary protective barrier" (see "Protective barrier").
"Protective barrier" means a barrier of radiation absorbing material(s) used to reduce radiation exposure. The types of protective barriers are as follows:
a. "Primary protective barrier" means the material, excluding filters, placed in the useful beam.
b. "Secondary protective barrier" means the material which attenuates stray radiation.
“Qualified expert” means an individual who has demonstrated to the satisfaction of the Agency that such individual possesses the knowledge and training to measure ionizing radiation, to evaluate safety techniques, and to advise regarding radiation protection needs, for example, individuals certified in the appropriate field by the American Board of Radiology, or the American Board of Health Physics, or the American Board of Medical Physics, or those having equivalent qualifications. With reference to the calibration of radiation therapy equipment, an individual, in addition to the above qualifications, must be qualified in accordance with 4492.3.3 and 4492.3.4.
"Qualified Medical Physicist" means an individual qualified in accordance with 3.3 and 3.4.
"Radiation detector" means a device which, in the presence of radiation provides, by either direct or indirect means, a signal or other indication suitable for use in measuring one or more quantities of incident radiation.
"Radiation field" (see "Useful beam").
"Radiation head" means the structure from which the useful beam emerges.
"Redundant beam monitoring system" means a combination of two independent dose monitoring systems in which each system is designed to terminate irradiation in accordance with a pre‑selected number of dose monitor units.
"Scattered radiation" means ionizing radiation emitted by interaction of ionizing radiation with matter, the interaction being accompanied by a change in direction of the radiation. Scattered primary radiation means that scattered radiation which has been deviated in direction only by materials irradiated by the useful beam.
"Secondary dose monitoring system" means a system which will terminate irradiation in the event of failure of the primary dose monitoring system.
"Secondary protective barrier" (see "Protective barrier").
"Shadow tray" means a device attached to the radiation head to support auxiliary beam blocking material.
"Shutter" means a device attached to the tube housing assembly which can totally intercept the useful beam and which has a lead equivalency not less than that of the tube housing assembly.
"Sievert (Sv)" means the SI unit of dose equivalent. The unit of dose equivalent is the joule per kilogram. The previous unit of dose equivalent (rem) is being replaced by the sievert. [1 Sv=100 rem.]
"Simulator (radiation therapy simulation system)" means any x‑ray system intended for localizing the volume to be exposed during radiation therapy and establishing the position and size of the therapeutic irradiation field. [See: Conventional Simulator and Virtual Simulator.]
"Source" means the region and/or material from which the radiation emanates.
"Source‑skin distance (SSD)" (see "Target‑skin distance").
"Stationary beam radiation therapy" means radiation therapy without displacement of one or more mechanical axes relative to the patient during irradiation.
"Stray radiation" means the sum of leakage and scattered radiation.
"Target" means that part of an x‑ray tube or accelerator onto which a beam of accelerated particles is directed to produce ionizing radiation or other particles.
"Target‑skin distance (TSD)" means the distance measured along the beam axis from the center of the front surface of the x‑ray target and/or electron virtual source to the surface of the irradiated object or patient.
"Tenth‑value layer (TVL)" means the thickness of a specified material which attenuates X‑radiation or gamma radiation to an extent such that the air kerma rate, exposure rate, or absorbed dose rate is reduced to one‑tenth of the value measured without the material at the same point.
"Termination of irradiation" means the stopping of irradiation in a fashion which will not permit continuance of irradiation without the resetting of operating conditions at the control panel.
"Therapeutic radiation machine" means x‑ray or electron‑producing equipment designed and used for external beam radiation therapy. For the purpose of these regulations, devices used to administer electronic brachytherapy shall also be considered therapeutic radiation machines.
"Tube" means an x‑ray tube, unless otherwise specified.
"Tube housing assembly" means the tube housing with tube installed. It includes high‑voltage and/or filament transformers and other appropriate elements when such are contained within the tube housing.
"Useful beam" means the radiation emanating from the tube housing port or the radiation head and passing through the aperture of the beam limiting device when the exposure controls are in a mode to cause the therapeutic radiation machine to produce radiation.
"Virtual Simulator" means a computed tomography (CT) unit used in conjunction with relevant software which recreates the treatment machine; and that allows import, manipulation, display, and storage of images from CT and/or other imaging modalities.
"Virtual source" means a point from which radiation appears to originate.
"Wedge filter" means a filter which effects continuous change in transmission over all or a part of the useful beam.
"Written directive" means an order in writing for the administration of radiation to a specific patient or human research subject, as specified in 5.1.
"X‑ray tube" means any electron tube which is designed to be used primarily for the production of x‑rays.
3.1 Administrative Controls. The registrant shall be responsible for directing the operation of the therapeutic radiation machines that have been registered with the Agency. The registrant or the registrant's agent shall ensure that the requirements of Part X are met in the operation of the therapeutic radiation machine(s).
3.2 A therapeutic radiation machine that does not meet the provisions of these regulations shall not be used for irradiation of patients.
3.3 Training for Therapeutic Radiation Machine Authorized Users. The registrant for any therapeutic radiation machine subject to 6.0 or 7.0 shall require the authorized user to be a physician who:
3.3.1 Is certified in:
3.3.1.1 Radiation oncology or therapeutic radiology by the American Board of Radiology or Radiology (combined diagnostic and therapeutic radiology program) by the American Board of Radiology prior to 1976; or
3.3.1.2 Radiation oncology by the American Osteopathic Board of Radiology; or
3.3.1.3 Radiology, with specialization in radiotherapy, as a British "Fellow of the Faculty of Radiology" or "Fellow of the Royal College of Radiology"; or
3.3.1.4 Therapeutic radiology by the Canadian Royal College of Physicians and Surgeons; or
3.3.2 Is in the active practice of therapeutic radiology, and has completed 200 hours of instruction in basic radiation techniques applicable to the use of an external beam radiation therapy unit, five hundred (500) hours of supervised work experience, and a minimum of three (3) years of supervised clinical experience.
3.3.2.1 To satisfy the requirement for instruction, the classroom and laboratory training shall include:
3.3.2.1.1 Radiation physics and instrumentation;
3.3.2.1.2 Radiation protection;
3.3.2.1.3 Mathematics pertaining to the use and measurement of ionization radiation; and
3.3.2.1.4 Radiation biology.
3.3.2.2 To satisfy the requirement for supervised work experience, training shall be under the supervision of an authorized user and shall include:
3.3.2.2.1 Review of the full calibration measurements and periodic quality assurance checks;
3.3.2.2.2 Evaluation of prepared treatment plans and calculation of treatment times/patient treatment settings;
3.3.2.2.3 Using administrative controls to prevent misadministrations;
3.3.2.2.4 Implementing emergency procedures to be followed in the event of the abnormal operation of an external beam radiation therapy unit or console; and
3.3.2.2.5 Checking and using radiation survey meters.
3.3.2.3 To satisfy the requirement for a period of supervised clinical experience, training shall include one (1) year in a formal training program approved by the Residency Review Committee for Radiology of the Accreditation Council for Graduate Medical Education or the Committee on Postdoctoral Training of the American Osteopathic Association and an additional two (2) years of clinical experience in therapeutic radiology under the supervision of an authorized user. The supervised clinical experience shall include:
3.3.2.3.1 Examining individuals and reviewing their case histories to determine their suitability for external beam radiation therapy treatment, and any limitations/contraindications;
3.3.2.3.2 Selecting proper dose and how it is to be administered;
3.3.2.3.3 Calculating the therapeutic radiation machine doses and collaborating with the authorized user in the review of patients' progress and consideration of the need to modify originally prescribed doses and/or treatment plans as warranted by patients' reaction to radiation; and
3.3.2.3.4 Post‑administration follow‑up and review of case histories.
3.3.3 Notwithstanding the requirements of 3.31 and 3.3.2 the registrant for any therapeutic radiation machine subject to 6.0 may also submit the training of the prospective authorized user physician for Agency review on a case‑by‑case basis.
3.3.4 A physician shall not act as an authorized user for any therapeutic radiation machine until such time as said physician's training has been reviewed and approved by the Agency.] 1*/
3.4 Training for Qualified Medical Physicist. The registrant for any therapeutic radiation machine subject to 6.0 or 7.0 shall require the Qualified Medical Physicist to:
3.4.1 Be registered with the Agency, under the provisions of Part B of these regulations, as a provider of radiation services in the area of calibration and compliance surveys of external beam radiation therapy units; and
3.4.2 Be certified by the American Board of Radiology in:
3.4.2.1 Therapeutic medical physics; or
3.4.2.2 Diagnostic medical physics; or
3.4.2.3 Nuclear medical physics; or
3.4.2.4 Radiological physics; or
3.4.2.5 Be certified by the American Board of Medical Physics in Radiation Oncology Physics; or
3.4.2.6 Be certified by the Canadian College of Medical Physics; or
3.4.2.7 Hold a master's or doctor's degree in physics, medical physics, other physical science, engineering, or applied mathematics from an accredited college or university, and have completed one (1) year of full time training in medical physics and an additional year of full time work experience under the supervision of a Qualified Medical Physicist at a medical institution. This training and work experience shall be conducted in clinical radiation facilities that provide high-energy external beam radiation therapy (photons and electrons with energies greater than or equal to one MV/one MeV). To meet this requirement, the individual shall have performed the tasks listed in 4.1, 6.16, 7.20 and 6.16, 7.21 under the supervision of a Qualified Medical Physicist during the year of work experience.
3.5 Qualifications of Operators.
3.5.1 Individuals who will be operating a therapeutic radiation machine for medical use shall be American Registry of Radiologic Technologists (ARRT) Registered Radiation Therapy Technologists. Individuals who are not ARRT Registered Radiation Therapy Technologists shall submit evidence that they have satisfactorily completed a radiation therapy technologist training program that complies with the requirements of the Joint Review Committee on Education in Radiologic Technology.3/
3.5.2 The names and training of all personnel currently operating a therapeutic radiation machine shall be kept on file at the facility. Information on former operators shall be retained for a period of at least two (2) years beyond the last date they were authorized to operate a therapeutic radiation machine at that facility.]
3.6 Written safety procedures and rules shall be developed by a Qualified Medical Physicist and shall be available in the control area of a therapeutic radiation machine, including any restrictions required for the safe operation of the particular therapeutic radiation machine. The operator shall be able to demonstrate familiarity with these rules.
3.7 Individuals shall not be exposed to the useful beam except for medical therapy purposes and unless such exposure has been ordered in writing by a therapeutic radiation machine authorized user. This provision specifically prohibits deliberate exposure of an individual for training, demonstration or other non‑healing‑arts purposes.
3.8 Visiting Authorized User. Notwithstanding the provisions of 3.7 a registrant may permit any physician to act as a visiting authorized user under the term of the registrant's Certificate of Registration for up to sixty (60) days per calendar year under the following conditions:
3.8.1 The visiting authorized user has the prior written permission of the registrant's management and, if the use occurs on behalf of an institution, the institution's Radiation Safety Committee (where applicable); and
3.8.2 The visiting authorized user meets the requirements established for authorized user(s) in 3.3.1 and 3.3.2 and
3.8.3 The registrant shall maintain copies of the written permission required in 3.8.1 and documentation that the visiting authorized user met the requirements of 3.8.2 for five (5) years from the date of the last visit.
3.9 All individuals associated with the operation of a therapeutic radiation machine shall be instructed in and shall comply with the provisions of the registrant's quality management program. In addition to the requirements of Part X, these individuals are also subject to the requirements of Parts D.1201, D.1502 and D.2104 of these regulations.
3.10 Information and Maintenance Record and Associated Information. The registrant shall maintain the following information in a separate file or package for each therapeutic radiation machine, for inspection by the Agency:
3.10.1 Report of acceptance testing;
3.10.2 Records of all surveys, calibrations, and periodic quality assurance checks of the therapeutic radiation machine required by Part X, as well as the name(s) of person(s) who performed such activities;
3.10.3 Records of maintenance and/or modifications performed on the therapeutic radiation machine, as well as the name(s) of person(s) who performed such services;
3.10.4 Signature of person authorizing the return of therapeutic radiation machine to clinical use after service, repair, or upgrade.
3.11 Records Retention. All records required by Part X shall be retained until disposal is authorized by the Agency unless another retention period is specifically authorized in Part X. All required records shall be retained in an active file from at least the time of generation until the next Agency inspection. Any required record generated prior to the last Agency inspection may be microfilmed or otherwise archived as long as a complete copy of said record can be retrieved until such time as the Agency authorizes final disposal.
4.1 Protection Surveys.
4.1.1 The registrant shall ensure that radiation protection surveys of all new facilities, and existing facilities not previously surveyed are performed with an operable radiation measurement survey instrument calibrated in accordance with 8.0. The radiation protection survey shall be performed by, or under the direction of, a Qualified Medical Physicist or a qualified expert and shall verify that, with the therapeutic radiation machine in a "BEAM‑ON" condition, with the largest clinically available treatment field and with a scattering phantom in the useful beam of radiation:
4.1.1.1 Radiation levels in restricted areas are not likely to cause personnel exposures in excess of the limits specified in Part D.1201a.of these regulations; and
4.1.1.2 Radiation levels in unrestricted areas do not exceed the limits specified in Parts D.1301a. and D.1301b. of these regulations.
4.1.2.1 After making any change in the treatment room shielding;
4.1.2.2 After making any change in the location of the therapeutic radiation machine within the treatment room;
4.1.2.3 After relocating the therapeutic radiation machine; or
4.1.2.4 Before using the therapeutic radiation machine in a manner that could result in increased radiation levels in areas outside the external beam radiation therapy treatment room.
4.1.3 The survey record shall indicate all instances where the facility, in the opinion of the Qualified Medical Physicist or a Qualified Expert, is in violation of applicable regulations. The survey record shall also include: the date of the measurements; the reason the survey is required; the manufacturer's name; model number and serial number of the therapeutic radiation machine; the instrument(s) used to measure radiation levels; a plan of the areas surrounding the treatment room that were surveyed; the measured dose rate at several points in each area expressed in microsieverts or millirems per hour; the calculated maximum level of radiation over a period of one (1) week for each restricted and unrestricted area; and the signature of the individual responsible for conducting the survey;
4.1.4 If the results of the surveys required by 4.1.1.1 or 4.1.1.2 indicate any radiation levels in excess of the respective limit specified in 4.1.1.1 the registrant shall lock the control in the "OFF" position and not use the unit:
4.1.4.1 Except as may be necessary to repair, replace, or test the therapeutic radiation machine, the therapeutic radiation machine shielding, or the treatment room shielding; or
4.1.4.2 Until the registrant has received a specific exemption from the Agency.
4.2 Modification of Radiation Therapy Unit or Room Before Beginning a Treatment Program. If the survey required by 4.1.1 indicates that an individual in an unrestricted area may be exposed to levels of radiation greater than those permitted by Parts D.1301a. and D.1301b. of these regulations, before beginning the treatment program the registrant shall:
4.2.1 Either equip the unit with beam direction interlocks or add additional radiation shielding to ensure compliance with Parts D.1301a. and D.1301b. of these regulations;
4.2.2 Perform the survey required by 4.1 again; and
4.2.3 Include in the report required by 4.4 the results of the initial survey, a description of the modification made to comply with 4.2.1 and the results of the second survey; or
4.2.4 Request and receive a registration amendment under Part D.1301c.of these regulations that authorizes radiation levels in unrestricted areas greater than those permitted by Parts D.1301a. and D.1301b. of these regulations.
4.3 Dosimetry Equipment.
4.3.1 The registrant shall have a calibrated dosimetry system available for use. The system shall have been calibrated by the National Institute for Standards and Technology (NIST) or by an American Association of Physicists in Medicine (AAPM) Accredited Dosimetry Calibration Laboratory (ADCL). The calibration shall have been performed within the previous twenty-four (24) months and after any servicing that may have affected system calibration. An independent survey shall be conducted by a qualified expert or Qualified Medical Physicist other than the person performing the original survey prior to the equipment being used except as described in X.4a.iv.
4.3.1.1 For beams with energies greater than 1 MV (1 MeV), the dosimetry system shall have been calibrated for Cobalt‑60;
4.3.1.2 For beams with energies equal to or less than 1 MV (1 MeV), the dosimetry system shall have been calibrated at an energy (energy range) appropriate for the radiation being measured;
4.3.2 The registrant shall have available for use a dosimetry system for quality assurance check measurements. To meet this requirement, the system may be compared with a system that has been calibrated in accordance with 4.3.1. This comparison shall have been performed within the previous twelve (12) months and after each servicing that may have affected system calibration. The quality assurance check system may be the same system used to meet the requirement in 4.3.1
4.4 Reports of External Beam Radiation Therapy Surveys and Measurements. The registrant for any therapeutic radiation machine subject to 6.0 or 7.0 shall furnish a copy of the records required in 4.1 and 4.2 to the Agency within thirty (30) days following completion of the action that initiated the record requirement.
Each registrant or applicant subject to 6.0, 7.0 or 11.0 shall develop, implement, and maintain a quality management program to provide high confidence that radiation will be administered as directed by the authorized user.
5.1 Scope and Applicability. The quality management program shall address, as a minimum, the following specific objectives:
5.1.1 Written Directives:
5.1.1.1 A written directive must be dated and signed by an authorized user prior to the administration of radiation.
If because of the patient’s condition, a delay in the order to provide a written revision to an existing written directive would jeopardize the patient’s health, an oral revision to an existing written directive will be acceptable, provided that the oral revision is documented as soon as possible in writing in the patient’s record and a revised written directive is signed by an authorized user within 48 hours of the oral revision.
5.1.1.2 The written directive must contain the patient or human research subject’s name, the type and energy of the beam, the total dose, dose per fraction, treatment site, and number of fractions.
5.1.1.3 A written revision to an existing written directive may be made provided that the revision is dated and signed by an authorized user prior to the administration of the therapeutic radiation machine dose, or the next fractional dose.
5.1.1.4 The registrant shall retain a copy of the written directive for three (3) years.
5.1.2 Procedures for Administrations. The registrant shall develop, implement, and maintain written procedures to provide high confidence that:
5.1.2.1 Prior to the administration of each course of radiation treatments, the patient’s or human research subject’s identity is verified by more than one method as the individual named in the written directive;
5.1.2.2 Each administration is in accordance with the written directive;
5.1.2.3 Therapeutic radiation machine final plans of treatment and related calculations are in accordance with the respective written directives by:
5.1.2.3.1 Checking both manual and computer generated dose calculations to verify they are correct and in accordance with the written directive; and
5.1.2.3.2 Verifying that any computer-generated calculations are correctly transferred into the consoles of authorized therapeutic medical units;
5.1.2.4 Any unintended deviation from the written directive is identified, evaluated and appropriate action is taken; and
5.1.2.5 The registrant retains a copy of the procedures for administrations for the duration of the registration.
5.2 Reports and Notifications of Misadministrations.
5.2.1 A registrant shall report any event resulting from intervention of a patient or human research subject in which the administration of therapeutic radiation machine radiation results, or will result in, unintended permanent functional damage to an organ or a physiological system as determined by a physician.
5.2.2 Other than events that result from intervention by a patient or human research subject, a registrant shall report any event in which the administration of a therapeutic radiation machine therapy dose:
5.2.2.1 Involves the wrong patient, wrong treatment modality, or wrong treatment site; or
5.2.2.2 The calculated weekly administered dose differs from the weekly prescribed dose by more than thirty percent (30%); or
5.2.2.3 The calculated total administered dose differs from the total prescribed dose by more than twenty percent (20%) of the total prescribed dose;
5.2.4.1 The registrant’s name;
5.2.4.2 The name of the prescribing physician;
5.2.4.3 A brief description of the event;
5.2.4.4 Why the event occurred;
5.2.4.5 The effect, if any, on the individuals(s) who received the administration;
5.2.4.6 Actions, if any, that have been taken, or are planned, to prevent recurrence;
5.2.4.7 Certification that the registrant notified the individual (or the individual’s responsible relative or guardian), and if not, why not.
5.2.5 The report shall not contain the individual’s name or any other information that could lead to the identification of the individual.
5.2.6 The registrant shall provide notification of the event to the referring physician and also notify the individual who is the subject of the misadministration no later than twenty-four (24) hours after its discovery, unless the referring physician personally informs the registrant either that he or she will inform the individual or that, based on medical judgment, telling the individual would be harmful. The registrant is not required to notify the individual without first consulting the referring physician. If the referring physician or the affected individual cannot be reached within twenty-four (24) hours, the registrant shall notify the individual as soon as possible thereafter. The registrant may not delay any appropriate medical care for the individual, including any necessary remedial care as a result of the misadministration, because of any delay in notification. To meet the requirements of this paragraph, the notification of the individual who is the subject of the misadministration may be made instead to that individual’s responsible relative or guardian. If a verbal notification is made, the registrant shall inform the individual, or appropriate responsible relative or guardian, that a written description of the event can be obtained from the registrant upon request. The registrant shall provide such a written description if requested.
5.2.7 Aside from the notification requirement, nothing in this section affects any rights or duties of registrants and physicians in relation to each other, to individuals affected by the misadministration, or to that individual’s responsible relatives or guardians.
5.2.8 The registrant shall retain a record of a misadministration in accordance with 5.3. A copy of the record required shall be provided to the referring physician if other than the registrant within fifteen (15) days after discovery of the misadministration.
5.3 Records of Misadministrations. A registrant shall retain a record of misadministrations reported in accordance with 5.2 for three (3) years. The record must contain the following:
5.3.1 The registrant’s name and the names of the individuals involved;
5.3.2 The social security number or other identification number, if one has been assigned, of the individual who is the subject of the misadministration;
5.3.3 A brief description of the event; why it occurred; the effect, if any, on the individual;
5.3.4 The actions, if any, taken or planned to prevent recurrence; and
5.3.5 Whether the registrant notified the individual (or the individual’s responsible relative or guardian) and, if not, whether such failure to notify was based on guidance from the referring physician.
6.1 Leakage Radiation. When the x‑ray tube is operated at its maximum rated tube current for the maximum kV, the leakage air kerma rate shall not exceed the value specified at the distance specified for that classification of therapeutic radiation machine:
6.1.1 5‑50 kV Systems. The leakage air kerma rate measured at any position 5 centimeters from the tube housing assembly shall not exceed 1 mGy (100 mrad) in any one hour.
6.1.2 >50 and <500 kV Systems. The leakage air kerma rate measured at a distance of 1 meter from the target in any direction shall not exceed 1 cGy (1 rad) in any 1 hour. This air kerma rate measurement may be averaged over areas no larger than one hundred square centimeters (100 cm2). In addition, the air kerma rate at a distance of 5 centimeters from the surface of the tube housing assembly shall not exceed 30 cGy (30 rad) per hour.
6.1.3 For each therapeutic radiation machine, the registrant shall determine, or obtain from the manufacturer, the leakage radiation existing at the positions specified in 6.1.1 and 6.1.2 for the specified operating conditions. Records on leakage radiation measurements shall be maintained at the installation for inspection by the Agency.
6.2 Permanent Beam Limiting Devices. Permanent diaphragms or cones used for limiting the useful beam shall provide at least the same degree of attenuation as required for the tube housing assembly.
6.3 Adjustable or Removable Beam Limiting Devices.
6.3.1 All adjustable or removable beam limiting devices, diaphragms, cones or blocks shall not transmit more than 5 percent of the useful beam for the most penetrating beam used;
6.3.2 When adjustable beam limiting devices are used, the position and shape of the radiation field shall be indicated by a light beam.
6.4 Filter System. The filter system shall be so designed that:
6.4.1 Filters can not be accidentally displaced at any possible tube orientation;
6.4.2 For equipment installed after July 10, 2002 an interlock system prevents irradiation if the proper filter is not in place;
6.4.3 The air kerma rate escaping from the filter slot shall not exceed 1 cGy (1 rad) per hour at one (1) meter under any operating conditions; and
6.4.4 Each filter shall be marked as to its material of construction and its thickness.
6.5 Tube Housing.
6.5.1 The x‑ray tube shall be so mounted that it can not accidentally turn or slide with respect to the housing aperture; and
6.5.2 The tube housing assembly shall be capable of being immobilized for stationary portal treatments.
6.5.3 Source Marking. The tube housing assembly shall be so marked that it is possible to determine the location of the source to within 5 millimeters, and such marking shall be readily accessible for use during calibration procedures.
6.6 Beam Block. Contact therapy tube housing assemblies shall have a removable shield of material, equivalent in attenuation to 0.5 millimeters of lead at 100 kV, which can be positioned over the entire useful beam exit port during periods when the beam is not in use.
6.7 Timer. A suitable irradiation control device shall be provided to terminate the irradiation after a pre‑set time interval.
6.7.1 A timer with a display shall be provided at the treatment control panel. The timer shall have a pre‑set time selector and an elapsed time or time remaining indicator;
6.7.2 The timer shall be a cumulative timer that activates with an indication of "BEAM‑ON" and retains its reading after irradiation is interrupted or terminated. After irradiation is terminated and before irradiation can be reinitiated, it shall be necessary to reset the elapsed time indicator;
6.7.3 The timer shall terminate irradiation when a pre‑selected time has elapsed, if any dose monitoring system present has not previously terminated irradiation;
6.7.4 The timer shall permit accurate pre‑setting and determination of exposure times as short as 1 second;
6.7.5 The timer shall not permit an exposure if set at zero;
6.7.6 The timer shall not activate until the shutter is opened when irradiation is controlled by a shutter mechanism unless calibration includes a timer error correction to compensate for mechanical lag; and
6.7.7 Timer shall be accurate to within 1 percent of the selected value or 1 second, whichever is greater.
6.8 Control Panel Functions. The control panel, in addition to the displays required by other provisions in 6.0 shall have:
6.8.1 An indication of whether electrical power is available at the control panel and if activation of the x‑ray tube is possible;
6.8.2 An indication of whether x‑rays are being produced;
6.8.3 A means for indicating x‑ray tube potential and current;
6.8.4 The means for terminating an exposure at any time;
6.8.5 A locking device which will prevent unauthorized use of the therapeutic radiation machine; and
6.8.6 For therapeutic radiation machines manufactured after July 10, 2002, a positive display of specific filter(s) in the beam.
6.9 Multiple Tubes. When a control panel may energize more than one x‑ray tube:
6.9.1 It shall be possible to activate only one x‑ray tube at any time;
6.9.2 There shall be an indication at the control panel identifying which x‑ray tube is activated; and
6.9.3 There shall be an indication at the tube housing assembly when that tube is energized.
6.10 Target‑to‑Skin Distance (TSD). There shall be a means of determining the central axis TSD to within one (1) centimeter and of reproducing this measurement to within two (2) millimeters thereafter.
6.11 Shutters. Unless it is possible to bring the x‑ray output to the prescribed exposure parameters within 5 seconds after the x‑ray "ON" switch is energized, the beam shall be attenuated by a shutter having a lead equivalency not less than that of the tube housing assembly. In addition, after the unit is at operating parameters, the shutter shall be controlled by the operator from the control panel. An indication of shutter position shall appear at the control panel.
6.12 Low Filtration X‑ray Tubes. Each therapeutic radiation machine equipped with a beryllium or other low‑filtration window shall be clearly labeled as such upon the tube housing assembly and shall be provided with a permanent warning device on the control panel that is activated when no additional filtration is present, to indicate that the dose rate is very high.
6.13 Facility Design Requirements for Therapeutic Radiation Machines Capable of Operating in the Range 50 kV to 500 kV. In addition to shielding adequate to meet requirements of 9.0, the treatment room shall meet the following design requirements:
6.13.1 Aural Communication. Provision shall be made for continuous two‑way aural communication between the patient and the operator at the control panel;
6.13.2 Viewing Systems. Provision shall be made to permit continuous observation of the patient during irradiation and the viewing system shall be so located that the operator can observe the patient from the control panel. The therapeutic radiation machine shall not be used for patient irradiation unless at least one viewing system is operational.
6.14 Additional Requirements. Treatment rooms that contain a therapeutic radiation machine capable of operating above 150 kV shall meet the following additional requirements:
6.14.1 All protective barriers shall be fixed except for entrance doors or beam interceptors;
6.14.2 The control panel shall be located outside the treatment room or in a totally enclosed booth, which has a ceiling, inside the room;
6.14.3 Interlocks shall be provided such that all entrance doors, including doors to any interior booths, shall be closed before treatment can be initiated or continued. If the radiation beam is interrupted by any door opening, it shall not be possible to restore the machine to operation without closing the door and reinitiating irradiation by manual action at the control panel; and
6.14.4 When any door referred to in 6.14.3 is opened while the x‑ray tube is activated, the air kerma rate at a distance of 1 meter from the source shall be reduced to less than 1 mGy (100 mrad) per hour.
6.15 Full Calibration Measurements.
6.15.1 Full calibration of a therapeutic radiation machine subject to 6.0 shall be performed by, or under the direct supervision of, a Qualified Medical Physicist:
6.15.1.1 Before the first medical use following installation or reinstallation of the therapeutic radiation machine;
6.15.1.2 At intervals not exceeding one (1) year; and
6.15.1.3 Before medical use under the following conditions:
6.15.1.3.1 Whenever quality assurance check measurements indicate that the radiation output differs by more than five percent (5%) from the value obtained at the last full calibration and the difference cannot be reconciled; and
6.15.1.3.2 Following any component replacement, major repair, or modification of components that could significantly affect the characteristics of the radiation beam.
6.15.1.4 Notwithstanding the requirements of 6.15.1.3:
6.15.1.4.1 Full calibration of therapeutic radiation machines with multi‑energy capabilities is required only for those modes and/or energies that are not within their acceptable range; and
6.15.1.4.2 If the repair, replacement or modification does not affect all energies, full calibration shall be performed on the affected energy that is in most frequent clinical use at the facility. The remaining energies may be validated with quality assurance check procedures against the criteria in 6.15.1.4.1
6.16 Periodic Quality Assurance Checks.
6.16.1 Periodic quality assurance checks shall be performed on therapeutic radiation machines subject to 6.0 which are capable of operation at greater than or equal to 50 kV.
6.16.2 To satisfy the requirement of 6.16.1 quality assurance checks shall meet the following requirements:
6.16.2.1 The registrant shall perform quality assurance checks in accordance with written procedures established by the Qualified Medical Physicist; and
6.16.2.2 The quality assurance check procedures shall specify the frequency at which tests or measurements are to be performed. The quality assurance check procedures shall specify that the quality assurance check shall be performed during the calibration specified in 6.15.1. The acceptable tolerance for each parameter measured in the quality assurance check, when compared to the value for that parameter determined in the calibration specified in 6.15.1 shall be stated.
6.16.3 The cause for a parameter exceeding a tolerance set by the Qualified Medical Physicist shall be investigated and corrected before the system is used for patient irradiation;
6.16.4 Whenever a quality assurance check indicates a significant change in the operating characteristics of a system, as specified in the Qualified Medical Physicist's quality assurance check procedures, the system shall be recalibrated as required in 6.15.1
6.16.5 The registrant shall use the dosimetry system described in 4.3.2 to make the quality assurance check required in 6.15.2;
6.16.6 The registrant shall have the Qualified Medical Physicist review and sign the results of each radiation output quality assurance check within thirty (30) days of the date that the check was performed;
6.16.7 The registrant shall ensure that safety quality assurance checks of therapeutic radiation machines subject to 6.0 are performed at intervals not to exceed thirty (30) days;
6.16.8 Notwithstanding the requirements of 6.16.6 and 6.16.7, the registrant shall ensure that no therapeutic radiation machine is used to administer radiation to humans unless the quality assurance checks required by 6.16.6 and 6.16.7 have been performed within the thirty (30) day period immediately prior to said administration;
6.16.9 To satisfy the requirement of 6.16.7 safety quality assurance checks shall ensure proper operation of:
6.16.9.1 Electrical interlocks at each external beam radiation therapy room entrance;
6.16.9.2 The "BEAM‑ON" and termination switches;
6.16.9.3 Beam condition indicator lights on the access door(s), control console, and in the radiation therapy room;
6.16.9.4 Viewing systems;
6.16.9.5 If applicable, electrically operated treatment room doors from inside and outside the treatment room;
6.16.10 The registrant shall maintain a record of each quality assurance check required by 6.16.1 and 6.16.7 for 3 years. The record shall include: the date of the quality assurance check; the manufacturer's name, model number, and serial number of the therapeutic radiation machine; the manufacturer's name; model number and serial number for the instrument(s) used to measure the radiation output of the therapeutic radiation machine; and the signature of the individual who performed the periodic quality assurance check
6.17 Operating Procedures.
6.17.1 The therapeutic radiation machine shall not be used for irradiation of patients unless the requirements of 6.15 and 6.16 have been met;
6.17.2 Therapeutic radiation machines shall not be left unattended unless secured pursuant to 6.7.5
6.17.3 When a patient must be held in position for radiation therapy, mechanical supporting or restraining devices shall be used;
6.17.4 The tube housing assembly shall not be held by an individual during operation unless the assembly is designed to require such holding and the peak tube potential of the system does not exceed 50 kV. In such cases, the holder shall wear protective gloves and apron of not less than 0.5 millimeters lead equivalency at 100 kV;
6.17.5 A copy of the current operating and emergency procedures shall be maintained at the therapeutic radiation machine control console; and
6.17.6 No individual other than the patient shall be in the treatment room during exposures from therapeutic radiation machines operating above 150 kV. At energies less than or equal to 150 kV, any individual, other than the patient, in the treatment room shall be protected by a barrier sufficient to meet the requirements of Part D.1201 of these regulations.
6.18 Possession of Survey Instrument(s). Each facility location authorized to use a therapeutic radiation machine in accordance with 6.0 shall possess appropriately calibrated portable monitoring equipment.
6.18.1 As a minimum, such equipment shall include a portable radiation measurement survey instrument capable of measuring dose rates over the range 10 μSv (1 mrem) per hour to 10 mSv (1000 mrem) per hour. The survey instrument(s) shall be operable and calibrated in accordance with 8.0
7.1 Possession of Survey Instrument(s). Each facility location authorized to use a therapeutic radiation machine in accordance with 7.0 shall possess appropriately calibrated portable monitoring equipment.
7.1.1 As a minimum, such equipment shall include a portable radiation measurement survey instrument capable of measuring dose rates over the range 10 µSv (1 mrem) per hour to 10 mSv (1000 mrem) per hour. The survey instrument(s) shall be operable and calibrated in accordance with 8.0
7.2 Leakage Radiation Outside the Maximum Useful Beam in Photon and Electron Modes.
7.2.1 The absorbed dose due to leakage radiation (excluding neutrons) at any point outside the maximum sized useful beam, but within a circular plane of radius two (2) meters which is perpendicular to and centered on the central axis of the useful beam at the nominal treatment distance (i.e. patient plane), shall not exceed a maximum of 0.2 percent and an average of 0.1 percent of the absorbed dose on the central axis of the beam at the nominal treatment distance. Measurements shall be averaged over an area not exceeding one hundred square centimeters (100 cm2) at a minimum of sixteen (16) points uniformly distributed in the plane;
7.2.2 Except for the area defined in 7.2.1 the absorbed dose due to leakage radiation (excluding neutrons) at 1 meter from the electron path between the electron source and the target or electron window shall not exceed 0.5 percent of the absorbed dose on the central axis of the beam at the nominal treatment distance. Measurements shall be averaged over an area not exceeding one hundred square centimeters(100 cm2);
7.2.3 For equipment manufactured after July 10, 2002 the neutron absorbed dose outside the useful beam shall be in compliance with International Electrotechnical Commission (IEC) Document 60601‑2‑1 (most current revision); and
7.2.4 For each therapeutic radiation machine, the registrant shall determine, or obtain from the manufacturer, the leakage radiation existing at the positions specified in 7.2.1 through 7.2.3 for the specified operating conditions. Records on leakage radiation measurements shall be maintained at the installation for inspection by the Agency.
7.3 Leakage Radiation Through Beam Limiting Devices.
7.3.1 Photon Radiation. All adjustable or interchangeable beam limiting devices shall attenuate the useful beam such that at the nominal treatment distance, the maximum absorbed dose anywhere in the area shielded by the beam limiting device(s) shall not exceed 2 percent of the maximum absorbed dose on the central axis of the useful beam measured in a 100 cm2 radiation field, or maximum available field size if less than 100 cm2;
7.3.2 Electron Radiation. All adjustable or interchangeable electron applicators shall attenuate the radiation, including but not limited to photon radiation generated by electrons incident on the beam limiting device and electron applicator and other parts of the radiation head, such that the absorbed dose in a plane perpendicular to the central axis of the useful beam at the nominal treatment distance shall not exceed:
7.3.2.1 A maximum of two percent (2%) and average of 0.5 percent of the absorbed dose on the central axis of the useful beam at the nominal treatment distance. This limit shall apply beyond a line seven (7) centimeters outside the periphery of the useful beam; and
7.3.2.2 A maximum of ten percent (10%) of the absorbed dose on the central axis of the useful beam at the nominal treatment distance. This limit shall apply beyond a line two (2) centimeters outside the periphery of the useful beam.
7.3.2.3 Measurement of Leakage Radiation.
7.3.2.3.1 Photon Radiation. Measurements of leakage radiation through the beam limiting devices shall be made with the beam limiting devices closed and any residual aperture blocked by at least two (2) tenth value layers of suitable absorbing material. In the case of overlapping beam limiting devices, the leakage radiation through each set shall be measured independently at the depth of maximum dose. Measurements shall be made using a radiation detector of area not exceeding ten square centimeters (10 cm2);
7.3.2.3.2 Electron Radiation. Measurements of leakage radiation through the electron applicators shall be made with the electron beam directed into the air and using a radiation detector of area up to but not exceeding one (1) square centimeter suitably protected against radiation which has been scattered from material beyond the radiation detector. Measurements shall be made using one (1) centimeter of water equivalent build up material.
7.4 Filters/Wedges.
7.4.1 Each wedge filter that is removable from the system shall be clearly marked with an identification number. For removable wedge filters, the nominal wedge angle shall appear on the wedge or wedge tray (if permanently mounted to the tray). If the wedge or wedge tray is significantly damaged, the wedge transmission factor shall be redetermined;
7.4.2 If the absorbed dose rate information required by 7.9 relates exclusively to operation with a field flattening filter or beam scattering foil in place, such foil or filter shall be removable only by the use of tools;
7.4.3 For equipment manufactured after July 10, 2002 which utilizes wedge filters, interchangeable field flattening filters, or interchangeable beam scattering foils:
7.4.3.1 Irradiation shall not be possible until a selection of a filter or a positive selection to use "no filter" has been made at the treatment control panel, either manually or automatically;
7.4.3.2 An interlock system shall be provided to prevent irradiation if the filter selected is not in the correct position;
7.4.3.3 A display shall be provided at the treatment control panel showing the wedge filter(s), interchangeable field flattening filter(s), and/or interchangeable beam scattering foil(s) in use; and
7.4.3.4 An interlock shall be provided to prevent irradiation if any filter and/or beam scattering foil selection operation carried out in the treatment room does not agree with the filter and/or beam scattering foil selection operation carried out at the treatment control panel.
7.5 Stray Radiation in the Useful Beam. For equipment manufactured after July 10, 2002, the registrant shall determine during acceptance testing, or obtain from the manufacturer, data sufficient to ensure that x‑ray stray radiation in the useful electron beam, absorbed dose at the surface during x‑ray irradiation and stray neutron radiation in the useful x‑ray beam are in compliance with International Electrotechnical Commission (IEC) Document 60601‑2‑1 (most current revision).
7.6 Beam Monitors. All therapeutic radiation machines subject to 7.0 shall be provided with redundant beam monitoring systems. The sensors for these systems shall be fixed in the useful beam during treatment to indicate the dose monitor unit rate.
7.6.1 Equipment manufactured after July 10, 2002 shall be provided with at least two (2) independently powered integrating dose meters. Alternatively, common elements may be used if the production of radiation is terminated upon failure of any common element.
7.6.2 Equipment manufactured on or before July 10, 2002 shall be provided with at least one (1) radiation detector. This detector shall be incorporated into a useful beam monitoring system;
7.6.3 The detector and the system into which that detector is incorporated shall meet the following requirements:
7.6.3.1 Each detector shall be removable only with tools and, if movable, shall be interlocked to prevent incorrect positioning;
7.6.3.2 Each detector shall form part of a beam monitoring system from whose readings in dose monitor units the absorbed dose at a reference point can be calculated;
7.6.3.3 Each beam monitoring system shall be capable of independently monitoring, interrupting, and terminating irradiation; and
7.6.3.4 For equipment manufactured after July 10, 2002, the design of the beam monitoring systems shall ensure that the:
7.6.3.4.1 Malfunctioning of one system shall not affect the correct functioning of the other system(s); and
7.6.3.4.2 Failure of either system shall terminate irradiation or prevent the initiation of radiation.
7.6.3.5 Each beam monitoring system shall have a legible display at the treatment control panel. For equipment manufactured after July 10, 2002, each display shall:
7.6.3.5.1 Maintain a reading until intentionally reset;
7.6.3.5.2 Have only one scale and no electrical or mechanical scale multiplying factors;
7.6.3.5.3 Utilize a design such that increasing dose is displayed by increasing numbers; and
7.6.3.5.4 In the event of power failure, the beam monitoring information required in 7.6.3.5.3 displayed at the control panel at the time of failure shall be retrievable
7.7 Beam Symmetry.
7.7.1 A bent‑beam linear accelerator with beam flattening filter(s) subject to 7.0 shall be provided with auxiliary device(s) to monitor beam symmetry;
7.7.2 The device(s) referenced in 7.7.1 shall be able to detect field asymmetry greater than ten percent (10%); and
7.7.3 The device(s) referenced in 7.7.1 shall be configured to terminate irradiation if the specifications in 7.7.2 can not be maintained.
7.8 Selection and Display of Dose Monitor Units.
7.8.1 Irradiation shall not be possible until a new selection of a number of dose monitor units has been made at the treatment control panel;
7.8.2 The pre‑selected number of dose monitor units shall be displayed at the treatment control panel until reset manually for the next irradiation;
7.8.3 After termination of irradiation, it shall be necessary to reset the dosimeter display before subsequent treatment can be initiated; and
7.8.4 For equipment manufactured after July 10, 2002, after termination of irradiation, it shall be necessary for the operator to reset the pre‑selected dose monitor units before irradiation can be initiated.
7.9 Air Kerma Rate/Absorbed Dose Rate. For equipment manufactured after July 10, 2002, a system shall be provided from whose readings the air kerma rate or absorbed dose rate at a reference point can be calculated. [The radiation detectors specified in 7.6 may form part of this system.] In addition:
7.9.1 The dose monitor unit rate shall be displayed at the treatment control panel;
7.9.2 If the equipment can deliver under any conditions an air kerma rate or absorbed dose rate at the nominal treatment distance more than twice the maximum value specified by the manufacturer, a device shall be provided which terminates irradiation when the air kerma rate or absorbed dose rate exceeds a value twice the specified maximum. The dose rate at which the irradiation will be terminated shall be a record maintained by the registrant;
7.9.3 If the equipment can deliver under any fault condition(s) an air kerma rate or absorbed dose rate at the nominal treatment distance more than ten (10) times the maximum value specified by the manufacturer, a device shall be provided to prevent the air kerma rate or absorbed dose rate anywhere in the radiation field from exceeding twice the specified maximum value and to terminate irradiation if the excess absorbed dose at the nominal treatment distance exceeds 4 Gy (400 rad); and
7.9.4 For each therapeutic radiation machine, the registrant shall determine, or obtain from the manufacturer, the maximum value(s) specified in 7.9.2 and 7.9.3 for the specified operating conditions. Records of these maximum value(s) shall be maintained at the installation for inspection by the Agency.
7.10 Termination of Irradiation by the Beam Monitoring System or Systems During Stationary Beam Radiation Therapy.
7.10.1 Each primary system shall terminate irradiation when the pre‑selected number of dose monitor units has been detected by the system;
7.10.2 If the original design of the equipment included a secondary dose monitoring system, that system shall be capable of terminating irradiation when not more than fifteen percent (15%) or forty (40) dose monitor units above the pre‑selected number of dose monitor units set at the control panel has been detected by the secondary dose monitoring system; and
7.10.3 For equipment manufactured after July 10, 2002, an indicator on the control panel shall show which monitoring system has terminated irradiation.
7.11 Termination of Irradiation. It shall be possible to terminate irradiation and equipment movement or go from an interruption condition to termination condition at any time from the operator's position at the treatment control panel.
7.12 Interruption of Irradiation. If a therapeutic radiation machine has an interrupt mode, it shall be possible to interrupt irradiation and equipment movements at any time from the treatment control panel. Following an interruption it shall be possible to restart irradiation by operator action without any reselection of operating conditions. If any change is made of a pre‑selected value during an interruption, irradiation and equipment movements shall be automatically terminated.
7.13 Timer. A suitable irradiation control device shall be provided to terminate the irradiation after a pre‑set time interval.
7.13.1 A timer shall be provided which has a display at the treatment control panel. The timer shall have a pre‑set time selector and an elapsed time indicator;
7.13.2 The timer shall be a cumulative timer that activates with an indication of "BEAM‑ON" and retains its reading after irradiation is interrupted or terminated. After irradiation is terminated and before irradiation can be reinitiated, it shall be necessary to reset the elapsed time indicator;
7.13.3 The timer shall terminate irradiation when a pre‑selected time has elapsed, if the dose monitoring systems have not previously terminated irradiation.
7.14 Selection of Radiation Type. Equipment capable of both x‑ray therapy and electron therapy shall meet the following additional requirements:
7.14.1 Irradiation shall not be possible until a selection of radiation type (x‑rays or electrons) has been made at the treatment control panel;
7.14.2 The radiation type selected shall be displayed at the treatment control panel before and during irradiation;
7.14.3 An interlock system shall be provided to ensure that the equipment can principally emit only the radiation type that has been selected;
7.14.4 An interlock system shall be provided to prevent irradiation with x‑rays, except to obtain an image, when electron applicators are fitted;
7.14.5 An interlock system shall be provided to prevent irradiation with electrons when accessories specific for x‑ray therapy are fitted; and
7.14.6 An interlock system shall be provided to prevent irradiation if any selected operations carried out in the treatment room do not agree with the selected operations carried out at the treatment control panel.
7.15 Selection of Energy. Equipment capable of generating radiation beams of different energies shall meet the following requirements:
7.15.1 Irradiation shall not be possible until a selection of energy has been made at the treatment control panel;
7.15.2 The nominal energy value selected shall be displayed at the treatment control panel until reset manually for the next irradiation. After termination of irradiation, it shall be necessary to reset the nominal energy value selected before subsequent treatment can be initiated;
7.15.3 Irradiation shall not be possible until the appropriate flattening filter or scattering foil for the selected energy is in its proper location; and
7.15.4 For equipment manufactured after July 10, 2002, the selection of energy shall be in compliance with International Electrotechnical Commission (IEC) Document 60601‑2‑1 (most current revision).
7.16 Selection of Stationary Beam Radiation Therapy or Moving Beam Radiation Therapy. Therapeutic radiation machines capable of both stationary beam radiation therapy and moving beam radiation therapy shall meet the following requirements:
7.16.1 Irradiation shall not be possible until a selection of stationary beam radiation therapy or moving beam radiation therapy has been made at the treatment control panel;
7.16.2 The mode of operation shall be displayed at the treatment control panel;
7.16.3 An interlock system shall be provided to ensure that the equipment can operate only in the mode that has been selected;
7.16.4 An interlock system shall be provided to prevent irradiation if any selected parameter in the treatment room does not agree with the selected parameter at the treatment control panel;
7.16.5 Moving beam radiation therapy shall be controlled to obtain the selected relationships between incremental dose monitor units and incremental movement. For equipment manufactured after July 10, 2002:
7.16.5.1 An interlock system shall be provided to terminate irradiation if the number of dose monitor units delivered in any 10 degrees of rotation or 1 cm of linear motion differs by more than twenty percent (20%) from the selected value;
7.16.5.2 Where angle terminates the irradiation in moving beam radiation therapy, the dose monitor units delivered shall differ by less than five percent (5%) from the dose monitor unit value selected;
7.16.5.3 An interlock shall be provided to prevent motion of more than five (5) degrees or one (1) cm beyond the selected limits during moving beam radiation therapy;
7.16.5.4 An interlock shall be provided to require that a selection of direction be made at the treatment control panel in all units which are capable of both clockwise and counter‑clockwise moving beam radiation therapy.
7.16.5.5 Moving beam radiation therapy shall be controlled with both primary position sensors and secondary position sensors to obtain the selected relationships between incremental dose monitor units and incremental movement.
7.16.6 Where the beam monitor system terminates the irradiation in moving beam radiation therapy, the termination of irradiation shall be as required by 7.10 and
7.16.7 For equipment manufactured after July 10, 2002, an interlock system shall be provided to terminate irradiation if movement:
7.16.7.1 Occurs during stationary beam radiation therapy; or
7.16.7.2 Does not start or stops during moving beam radiation therapy unless such stoppage is a pre‑planned function.
7.17 Facility Design Requirements for Therapeutic Radiation Machines Operating above 500 kV. In addition to shielding adequate to meet requirements of 9.0 the following design requirements are made:
7.17.1 Protective Barriers. All protective barriers shall be fixed, except for access doors to the treatment room or movable beam interceptors;
7.17.2 Control Panel. In addition to other requirements specified in Part X, the control panel shall also:
7.17.2.1 Be located outside the treatment room;
7.17.2.2 Provide an indication of whether electrical power is available at the control panel and if activation of the radiation is possible;
7.17.2.3 Provide an indication of whether radiation is being produced; and
7.17.2.4 Include an access control (locking) device that will prevent unauthorized use of the therapeutic radiation machine;
7.17.3 Viewing Systems. Windows, mirrors, closed‑circuit television or an equivalent viewing system shall be provided to permit continuous observation of the patient following positioning and during irradiation and shall be so located that the operator may observe the patient from the treatment control panel. The therapeutic radiation machine shall not be used for patient irradiation unless at least one viewing system is operational;
7.17.4 Aural Communications. Provision shall be made for continuous two‑way aural communication between the patient and the operator at the control panel. The therapeutic radiation machine shall not be used for irradiation of patients unless continuous two‑way aural communication is possible;
7.17.5 Room Entrances. Treatment room entrances shall be provided with warning lights in a readily observable position near the outside of all access doors, which will indicate when the useful beam is "ON" and when it is "OFF";
7.17.6 Entrance Interlocks.Interlocks shall be provided such that all access controls are activated before treatment can be initiated or continued. If the radiation beam is interrupted by any access control, it shall not be possible to restore the machine to operation without resetting the access control and reinitiating irradiation by manual action at the control panel;
7.17.7 Beam Interceptor Interlocks. If the shielding material in any protective barrier requires the presence of a beam interceptor to ensure compliance with Parts D.1301a. and D.1301b. of these regulations, interlocks shall be provided to prevent the production of radiation, unless the beam interceptor is in place, whenever the useful beam is directed at the designated barrier(s);
7.17.8 Emergency Cutoff Switches. At least 1 emergency power cutoff switch shall be located in the radiation therapy room and shall terminate all equipment electrical power including radiation and mechanical motion. This switch is in addition to the termination switch required by 7.11. All emergency power cutoff switches shall include a manual reset so that the therapeutic radiation machine cannot be restarted from the unit's control console without resetting the emergency cutoff switch;
7.17.9 Safety Interlocks. All safety interlocks shall be designed so that any defect or component failure in the safety interlock system prevents or terminates operation of the therapeutic radiation machine; and
7.17.10 Surveys for Residual Radiation. Surveys for residual activity shall be conducted on all therapeutic radiation machines capable of generating photon and electron energies above 10 MV prior to machining, removing, or working on therapeutic radiation machine components which may have become activated due to photo‑neutron production.
7.18 Qualified Medical Physicist Support.
7.18.1 The services of a Qualified Medical Physicist shall be required in facilities having therapeutic radiation machines with energies of 500 kV and above. The Qualified Medical Physicist shall be responsible for:
7.18.1.1 Full calibration(s) required by 7.20 and protection surveys required by 4.1
7.18.1.2 Supervision and review of dosimetry;
7.18.1.3 Beam data acquisition and transfer for computerized dosimetry, and supervision of its use;
7.18.1.4 Quality assurance, including quality assurance check review required by 7.21.4
7.18.1.5 Consultation with the authorized user in treatment planning, as needed; and
7.18.1.6 Perform calculations/assessments regarding misadministrations.
7.18.2 If the Qualified Medical Physicist is not a full‑time employee of the registrant, the operating procedures required by 7.19 shall also specifically address how the Qualified Medical Physicist is to be contacted for problems or emergencies, as well as the specific actions, if any, to be taken until the Qualified Medical Physicist can be contacted.
7.19 Operating Procedures.
7.19.1 No individual, other than the patient, shall be in the treatment room during treatment or during any irradiation for testing or calibration purposes;
7.19.2 Therapeutic radiation machines shall not be made available for medical use unless the requirements of 4.1, 7.20 and 7.21 have been met;
7.19.3 Therapeutic radiation machines, when not in operation, shall be secured to prevent unauthorized use;
7.19.4 When adjustable beam limiting devices are used, the position and shape of the radiation field shall be indicated by a light field.
7.19.5 If a patient must be held in position during treatment, mechanical supporting or restraining devices shall be used; and
7.19.6 A copy of the current operating and emergency procedures shall be maintained at the therapeutic radiation machine control console.
7.20 Acceptance Testing, Commissioning and Full Calibration Measurements.
7.20.1 Acceptance testing, commissioning and full calibration of a therapeutic radiation machine subject to 7.0 shall be performed by, or under the direct supervision of, a Qualified Medical Physicist.
7.20.2 Acceptance testing and commissioning shall be performed in accordance with "AAPM Code of Practice for Radiotherapy Accelerators: AAPM Report No. 47", prepared by Radiation Therapy Task Group 45 and the manufacturer’s contractual specifications. Acceptance testing and commissioning shall be conducted before the first medical use following installation or reinstallation of the therapeutic radiation machine.
7.20.3 Full calibration shall include measurement of all applicable parameters required by Table II of "Comprehensive QA for Radiation Oncology: Report of AAPM Radiation Therapy: AAPM Report No. 46," prepared by Committee Task Group 40 and shall be performed in accordance with "AAPM Code of Practice for Radiotherapy Accelerators: AAPM Report No. 47" prepared by Radiation Therapy Task Group 45. Although it shall not be necessary to complete all elements of a full calibration at the same time, all applicable parameters (for all energies) shall be completed at intervals not exceeding twelve (12) calendar months, unless a more frequent interval is required in Table II.
7.20.4 The Qualified Medical Physicist shall perform all elements of a full calibration necessary to determine that all parameters are within acceptable limits:
7.20.4.1 Whenever quality assurance check measurements indicate that the radiation output differs by more than five percent (5%) from the value obtained at the last full calibration and the difference cannot be reconciled. Therapeutic radiation machines with multi‑energy and/or multi‑mode capabilities shall only require measurements for those modes and/or energies that are not within their acceptable range; and
7.20.4.2 Following any component replacement, major repair, or modification of components that could significantly affect the characteristics of the radiation beam. If the repair, replacement or modification does not affect all modes and/or energies, measurements shall be performed on the effected mode/energy that is in most frequent clinical use at the facility. The remaining energies/modes may be validated with quality assurance check procedures against the criteria in 7.20.4.1
7.20.5 The registrant shall maintain a record of each calibration in an auditable form for the duration of the registration. The record shall include: the date of the calibration; the manufacturer's name, model number and serial number for the therapeutic radiation machine; the model numbers and serial numbers of the instruments used to calibrate the therapeutic radiation machine; and the signature of the Qualified Medical Physicist responsible for performing the calibration.
7.21 Periodic Quality Assurance Checks.
7.21.1 Periodic quality assurance checks shall be performed on all therapeutic radiation machines subject to 7.0 at intervals not to exceed those specified in "Comprehensive QA for Radiation Oncology: AAPM Report No. 46,” prepared by AAPM Radiation Therapy Committee Task Group 40;
7.21.2 To satisfy the requirement of 7.21.1, quality assurance checks shall include determination of central axis radiation output and a representative sampling of periodic quality assurance checks contained in "Comprehensive QA for Radiation Oncology: AAPM Report No. 46 prepared by Radiation Therapy Committee Task Group 40. Representative sampling shall include all applicable referenced periodic quality assurance checks in an interval not to exceed twelve (12) consecutive calendar months;
7.21.3 The registrant shall use a dosimetry system that has been intercompared within the previous twelve (12) months with the dosimetry system described in 4.3.1 to make the periodic quality assurance checks required in 7.21.2
7.21.4 The registrant shall perform periodic quality assurance checks required by 7.21.1 in accordance with procedures established by the Qualified Medical Physicist;
7.21.5 The registrant shall review the results of each periodic radiation output check according to the following procedures:
7.21.5.1 The authorized user and Qualified Medical Physicist shall be immediately notified if any parameter is not within its acceptable tolerance. The therapeutic radiation machine shall not be made available for subsequent medical use until the Qualified Medical Physicist has determined that all parameters are within their acceptable tolerances;
7.21.5.2 If all quality assurance check parameters appear to be within their acceptable range, the quality assurance check shall be reviewed and signed by either the authorized user or Qualified Medical Physicist within 3 treatment days; and
7.21.5.3 The Qualified Medical Physicist shall review and sign the results of each radiation output quality assurance check at intervals not to exceed thirty (30) days.
7.21.6 Therapeutic radiation machines subject to 7.0 shall have applicable safety quality assurance checks listed in "Comprehensive QA for Radiation Oncology: AAPM Report No. 46," prepared by AAPM Radiation Therapy Committee Task Group 40 performed at intervals not to exceed 1 week;
7.21.7 To satisfy the requirement of 7.21.6 safety quality assurance checks shall ensure proper operation of:
7.21.7.1 Electrical interlocks at each external beam radiation therapy room entrance;
7.21.7.2 Proper operation of the "BEAM‑ON", interrupt and termination switches;
7.21.7.3 Beam condition indicator lights on the access doors, control console, and in the radiation therapy room;
7.21.7.4 Viewing systems;
7.21.7.5 Electrically operated treatment room door(s) from inside and outside the treatment room;
7.21.7.6 At least one emergency power cutoff switch. If more than one emergency power cutoff switch is installed and not all switches are tested at once, each switch shall be tested on a rotating basis. Safety quality assurance checks of the emergency power cutoff switches may be conducted at the end of the treatment day in order to minimize possible stability problems with the therapeutic radiation machine.
7.21.7.7 The registrant shall promptly repair any system identified in 7.21.7 that is not operating properly; and
7.21.8 The registrant shall maintain a record of each quality assurance check required by 7.21.1 and 7.21.7 for three (3) years.
7.21.8.1 The record shall include: the date of the quality assurance check; the manufacturer's name, model number, and serial number of the therapeutic radiation machine; the manufacturer's name, model number and serial number for the instrument(s) used to measure the radiation output of the therapeutic radiation machine; and the signature of the individual who performed the periodic quality assurance check.
7.21.9 Quality Assurance Checks for IMRT shall:
7.21.9.1 Include commissioning and testing of the treatment planning and delivery systems, routine quality assurance of the delivery system, and patient-specific validation of treatment plans;5/ and
7.21.9.2 Be performed in accordance with "Guidance document on delivery, treatment planning, and clinical implementation of IMRT: Report of the IMRT subcommittee of the AAPM radiation therapy committee: AAPM Report No. 82”; and
7.21.9.3 Be performed in accordance with the manufacturer’s contractual specifications.
8.1 The registrant shall ensure that the survey instruments used to show compliance with 4492 (Part X) have been calibrated before first use, at intervals not to exceed twelve (12) months, and following repair. The registrant shall check each survey instrument for consistent response with a dedicated check source before each use. The licensee is not required to keep records of these checks.
8.2 To satisfy the requirements of 8.1 the registrant shall:
8.2.1 Calibrate all required scale readings up to 10 mSv (1000 mrem) per hour with an appropriate radiation source that is traceable to the National Institute of Standards and Technology (NIST);
8.2.2 Calibrate at least two (2) points on each scale to be calibrated. These points should be at approximately 1/3 and 2/3 of full-scale; and
8.3 To satisfy the requirements of 8.2 the registrant shall:
8.3.1 Consider a point as calibrated if the indicated dose rate differs from the calculated dose rate by not more than 10 percent; and
8.3.2 Consider a point as calibrated if the indicated dose rate differs from the calculated dose rate by not more than 20 percent if a correction factor or graph is conspicuously attached to the instrument.
8.4 The registrant shall retain a record of each calibration required in 8.1 for three (3) years. The record shall include:
8.4.1 A description of the calibration procedure; and
8.4.2 A description of the source used and the certified dose rates from the source, and the rates indicated by the instrument being calibrated, the correction factors deduced from the calibration data, the signature of the individual who performed the calibration, and the date of calibration.
8.5 The registrant may obtain the services of individuals licensed by the Agency, the US Nuclear Regulatory Commission or an Agreement State to perform calibrations of survey instruments. Records of calibrations that contain information required by 8.4 shall be maintained by the registrant.
9.1 Each therapeutic radiation machine subject to 6.0 or 7.0 shall be provided with such primary and/or secondary barriers as are necessary to ensure compliance with Parts D.1201 and D.1301 of these regulations.
9.2 Facility design information for all new installations of a therapeutic radiation machine or installations of a therapeutic radiation machine of higher energy into a room not previously approved for that energy shall be submitted for Agency approval prior to actual installation of the therapeutic radiation machine. The minimum facility design information that must be submitted is contained in Appendix A to (Part X).
10.1 Quality assurance for a conventional or virtual simulator shall include acceptance testing and periodic verification of system performance; and
10.2 Be performed in accordance with "Comprehensive QA for Radiation Oncology: Report of AAPM Radiation Therapy Committee Task Group No.40: AAPM Report No. 46” for a conventional simulator; or
10.3 Be performed in accordance with “Quality assurance for computed tomography simulators and the computed tomography-simulation process: Report of the AAPM Radiation Therapy Committee Task Group No. 66: AAPM Report No. 83” for a virtual simulator.
11.1 Applicability. Electronic brachytherapy devices shall be subject to the requirements of 11.0 and shall be exempt for the requirements of 6.0.
11.1.1 An electronic brachytherapy device that does not meet the requirements of 11.0 shall not be used for irradiation of patients; and
11.1.2 An electronic brachytherapy device shall only be utilized for human use applications specifically approved by the U.S. Food and Drug Administration (FDA) unless participating in a research study approved by the registrant’s Institutional Review Board (IRB).
11.2 Possession of Survey Instrument(s). Each facility location authorized to use an electronic brachytherapy device in accordance with 11.0 shall possess appropriately calibrated portable monitoring equipment. As a minimum, such equipment shall include a portable radiation measurement survey instrument capable of measuring dose rates over the range 10 µSv (1 mrem) per hour to 10 mSv (1000 mrem) per hour. The survey instrument(s) shall be operable and calibrated in accordance with 8.0 for the applicable electronic brachytherapy source energy.
11.3 Facility Design Requirements for Electronic Brachytherapy Devices. In addition to shielding adequate to meet requirements of 9.0 the treatment room shall meet the following design requirements:
11.3.1 If applicable, provision shall be made to prevent simultaneous operation of more than one therapeutic radiation machine in a treatment room.
11.3.2 Access to the treatment room shall be controlled by a door at each entrance.
11.3.3 Each treatment room shall have provisions to permit continuous aural communication and visual observation of the patient from the treatment control panel during irradiation. The electronic brachytherapy device shall not be used for patient irradiation unless the patient can be observed.
11.3.4 For electronic brachytherapy devices capable of operating below 50 kV, radiation shielding for the staff in the treatment room shall be available, either as a portable shield and/or as localized shielded material around the treatment site.
11.3.5 For electronic brachytherapy devices capable of operating at greater than 150 kV: 6/
11.3.5.1 The control panel shall be located outside the treatment room; and
11.3.5.2 Electrical interlocks shall be provided for all door(s) to the treatment room that will:
11.3.5.2.1 Prevent the operator from initiating the treatment cycle unless each treatment room entrance door is closed;
11.3.5.2.2 Cause the source to be shielded when an entrance door is opened; and
11.3.5.2.3 Prevent the source from being exposed following an interlock interruption until all treatment room entrance doors are closed and the source on-off control is reset at the console.
11.4 Electrical Safety for Electronic Brachytherapy Devices.
11.4.1 The high voltage transformer shall be electrically isolated to prevent electrical and magnetic interference with the surrounding environment and ancillary equipment.
11.4.2 The high voltage transformer shall be isolated from personnel (e.g., operator) and the environment by a protective housing that can only be accessed through a cover requiring a tool for access or with electrical interlocks to prevent operation while open.
11.4.3 The high voltage transformer shall have appropriate safety labels warning personnel of potential electrical shock and/or heat related injuries.
11.4.4 Equipment manufactured after shall be in compliance with the most current revision of the following International Electrotechnical Commission (IEC) Documents:
11.4.4.1 IEC 60601-1:1998+A1+A2:1995;
11.4.4.2 IEC 60601-1-2:2001;
11.4.4.3 IEC 60601-2-8:1999; and
11.4.4.4 IEC 60601-2-17:2004.
11.5 Control Panel Functions. The control panel, in addition to the displays required by other provisions in 11.0 shall:
11.5.1 Provide an indication of whether electrical power is available at the control panel and if activation of the electronic brachytherapy source is possible;
11.5.2 Provide an indication of whether x‑rays are being produced;
11.5.3 Provide a means for indicating electronic brachytherapy source potential and current;
11.5.4 Provide the means for terminating an exposure at any time; and
11.5.5 Include an access control (locking) device that will prevent unauthorized use of the electronic brachytherapy device.
11.6 Timer. A suitable irradiation control device (timer) shall be provided to terminate the irradiation after a pre-set time interval or integrated charge on a dosimeter-based monitor.
11.6.1 A timer shall be provided at the treatment control panel. The timer shall indicate planed setting and the time elapsed or remaining;
11.6.2 The timer shall not permit an exposure if set at zero;
11.6.3 The timer shall be a cumulative device that activates with an indication of "BEAM-ON" and retains its reading after irradiation is interrupted or terminated. After irradiation is terminated and before irradiation can be reinitiated, it shall be necessary to reset the elapsed time indicator;
11.6.4 The timer shall terminate irradiation when a pre-selected time has elapsed, if any dose monitoring system has not previously terminated irradiation.
11.6.5 The timer shall permit setting of exposure times as short as 0.1 second; and
11.6.6 The timer shall be accurate to within one (1) percent of the selected value or 0.1 second, whichever is greater.
11.7 Qualified Medical Physicist Support.
11.7.1 The services of a Qualified Medical Physicist shall be required in facilities having electronic brachytherapy devices. The Qualified Medical Physicist shall be responsible for:
11.7.1.1 Evaluation of the output from the electronic brachytherapy source;
11.7.1.2 Generation of the necessary dosimetric information;
11.7.1.3 Supervision and review of treatment calculations prior to initial treatment of any treatment site;
11.7.1.4 Establishing the periodic and day-of-use quality assurance checks and reviewing the data from those checks as required in 11.11;
11.7.1.5 Consultation with the authorized user in treatment planning, as needed; and
11.7.1.6 Performing calculations/assessments regarding patient treatments that may constitute a misadministration.
11.7.2 If the Qualified Medical Physicist is not a full-time employee of the registrant, the operating procedures required by 11.8 shall also specifically address how the Qualified Medical Physicist is to be contacted for problems or emergencies, as well as the specific actions, if any, to be taken until the Qualified Medical Physicist can be contacted.
11.8 Operating Procedures.
11.8.1 Only individuals approved by the authorized user, Radiation Safety Officer, or Qualified Medical Physicist shall be present in the treatment room during treatment;
11.8.2 Electronic brachytherapy devices shall not be made available for medical use unless the requirements of 4.1, 11.9 and 11.10 have been met;
11.8.3 The electronic brachytherapy device shall be inoperable, either by hardware or password, when unattended by qualified staff or service personnel;
11.8.4 During operation, the electronic brachytherapy device operator shall monitor the position of all persons in the treatment room, and all persons entering the treatment room, to prevent entering persons from unshielded exposure from the treatment beam;
11.8.5 If a patient must be held in position during treatment, mechanical supporting or restraining devices shall be used;
11.8.6 Written procedures shall be developed, implemented, and maintained for responding to an abnormal situation. These procedures shall include:
11.8.6.1 Instructions for responding to equipment failures and the names of the individuals responsible for implementing corrective actions; and
11.8.6.2 The names and telephone numbers of the authorized users, the Qualified Medical Physicist, and the Radiation Safety Officer to be contacted if the device or console operates abnormally.
11.8.7 A copy of the current operating and emergency procedures shall be physically located at the electronic brachytherapy device control console7/;
11.8.8 Instructions shall be posted at the electronic brachytherapy device control console5/ to inform the operator of the names and telephone numbers of the authorized users, the Qualified Medical Physicist, and the Radiation Safety Officer to be contacted if the device or console operates abnormally; and
11.8.9 The Radiation Safety Officer, or his/her designee, and an authorized user shall be notified as soon as possible if the patient has a medical emergency, suffers injury or dies. The Radiation Safety Officer or the Qualified Medical Physicist shall inform the manufacturer of the event.
11.9 Safety Precautions for Electronic Brachytherapy Devices.
11.9.1 A Qualified Medical Physicist shall determine which persons in the treatment room require monitoring when the beam is energized;
11.9.2 An authorized user and a Qualified Medical Physicist shall be physically present during the initiation of all patient treatments involving the electronic brachytherapy device;
11.9.3 A Qualified Medical Physicist and either an authorized user or a physician or electronic brachytherapy device operator, under the supervision of an authorized user, who has been trained in the operation and emergency response for the electronic brachytherapy device, shall be physically present during continuation of all patient treatments involving the electronic brachytherapy device;
11.9.4 When shielding is required by 11.3.4, the electronic brachytherapy device operator shall use a survey meter to verify proper placement of the shielding immediately upon initiation of treatment. Alternatively, a Qualified Medical Physicist shall designate shield locations sufficient to meet the requirements of D.1201 of these regulations for any individual, other than the patient, in the treatment room; and
11.9.5 All personnel in the treatment room are required to remain behind shielding during treatment. A Qualified Medical Physicist shall approve any deviation from this requirement and shall designate alternative radiation safety protocols, compatible with patient safety, to provide an equivalent degree of protection.
11.10 Electronic Brachytherapy Source Calibration Measurements.
11.10.1 Calibration of the electronic brachytherapy source output for an electronic brachytherapy device subject to 11.0 shall be performed by, or under the direct supervision of, a Qualified Medical Physicist;
11.10.2 Calibration of the electronic brachytherapy source output shall be made for each electronic brachytherapy source, or after any repair affecting the x-ray beam generation, or when indicated by the electronic brachytherapy source quality assurance checks;
11.10.3 Calibration of the electronic brachytherapy source output shall utilize a dosimetry system described in 4.3;
11.10.4 Calibration of the electronic brachytherapy source output shall include, as applicable, determination of:
11.10.4.1 The output within two percent (2%) of the expected value, if applicable, or determination of the output if there is no expected value;
11.10.4.2 Timer accuracy and linearity over the typical range of use;
11.10.4.3 Proper operation of back-up exposure control devices;
11.10.4.4 Evaluation that the relative dose distribution about the source is within five percent (5%) of that expected; and
11.10.4.5 Source positioning accuracy to within one (1) millimeter within the applicator;
11.10.5 Calibration of the x-ray source output required by 11.10 through 11.10.4 shall be in accordance with current published recommendations from a recognized national professional association with expertise in electronic brachytherapy (when available). In the absence of a calibration protocol published by a national professional association, the manufacturer’s calibration protocol shall be followed.
11.10.6 The registrant shall maintain a record of each calibration in an auditable form for the duration of the registration. The record shall include: the date of the calibration; the manufacturer's name, model number and serial number for the electronic brachytherapy device and a unique identifier for it’s electronic brachytherapy source; the model numbers and serial numbers of the instrument(s) used to calibrate the electronic brachytherapy device; and the name and signature of the Qualified Medical Physicist responsible for performing the calibration.
11.11 Periodic and Day-of-Use Quality Assurance Checks for Electronic Brachytherapy Devices.
11.11.1 Quality assurance checks shall be performed on each electronic brachytherapy device subject to 11.0
11.11.1.1 At the beginning of each day of use;
11.11.1.2 Each time the device is moved to a new room or site8; and
11.11.1.3 After each x-ray tube installation.
11.11.2 The registrant shall perform periodic quality assurance checks required by 11.11.1 in accordance with procedures established by the Qualified Medical Physicist;
11.11.3 To satisfy the requirements of 11.11.1, radiation output quality assurance checks shall include as a minimum:
11.11.3.1 Verification that output of the electronic brachytherapy source falls within three percent (3%) of expected values, as appropriate for the device, as determined by:
11.11.3.1.1 Output as a function of time, or
11.11.3.1.2 Output as a function of setting on a monitor chamber.
11.11.3.2 Verification of the consistency of the dose distribution to within three percent (3%) of that found during calibration required by 11.10; and
11.11.3.3 Validation of the operation of positioning methods to ensure that the treatment dose exposes the intended location within one (1) mm; and
11.11.4 The registrant shall use a dosimetry system that has been intercompared within the previous twelve (12) months with the dosimetry system described in 4.3.1 to make the quality assurance checks required in 11.11.3;
11.11.5 The registrant shall review the results of each radiation output quality assurance check according to the following procedures:
11.11.5.1 An authorized user and Qualified Medical Physicist shall be immediately notified if any parameter is not within its acceptable tolerance. The electronic brachytherapy device shall not be made available for subsequent medical use until the Qualified Medical Physicist has determined that all parameters are within their acceptable tolerances;
11.11.5.2 If all radiation output quality assurance check parameters appear to be within their acceptable range, the quality assurance check shall be reviewed and signed by either the authorized user or Qualified Medical Physicist within two (2) days; and
11.11.5.3 The Qualified Medical Physicist shall review and sign the results of each radiation output quality assurance check at intervals not to exceed thirty (30) days.
11.11.6 To satisfy the requirements of 11.11.1, safety device quality assurance checks shall, at a minimum, assure:
11.11.6.1 Proper operation of radiation exposure indicator lights on the electronic brachytherapy device and on the control console;
11.11.6.2 Proper operation of viewing and intercom systems in each electronic brachytherapy facility, if applicable;
11.11.6.3 Proper operation of radiation monitors, if applicable;
11.11.6.4 The integrity of all cables, catheters or parts of the device that carry high voltages; and
11.11.6.5 Connecting guide tubes, transfer tubes, transfer-tube-applicator interfaces, and treatment spacers are free from any defects that interfere with proper operation.
11.11.7 If the results of the safety device quality assurance checks required in 11.11.5 indicate the malfunction of any system, a registrant shall secure the control console in the OFF position and not use the electronic brachytherapy device except as may be necessary to repair, replace, or check the malfunctioning system.
11.11.8 The registrant shall maintain a record of each quality assurance check required by 11.11.3 and 11.11.7 in an auditable form for three (3) years.
11.11.8.1 The record shall include the date of the quality assurance check; the manufacturer's name, model number and serial number for the electronic brachytherapy device; the name and signature of the individual who performed the periodic quality assurance check and the name and signature of the Qualified Medical Physicist who reviewed the quality assurance check;
11.11.8.2 For radiation output quality assurance checks required by 11.11.3 the record shall also include the unique identifier for the electronic brachytherapy source and the manufacturer's name; model number and serial number for the instrument(s) used to measure the radiation output of the electronic brachytherapy device.
11.12 Therapy-Related Computer Systems. The registrant shall perform acceptance testing on the treatment planning system of electronic brachytherapy-related computer systems in accordance with current published recommendations from a recognized national professional association with expertise in electronic brachytherapy (when available). In the absence of an acceptance testing protocol published by a national professional association, the manufacturer’s acceptance testing protocol shall be followed.
11.12.1 Acceptance testing shall be performed by, or under the direct supervision of, a Qualified Medical Physicist. At a minimum, the acceptance testing shall include, as applicable, verification of:
11.12.1.1 The source-specific input parameters required by the dose calculation algorithm;
11.12.1.2 The accuracy of dose, dwell time, and treatment time calculations at representative points;
11.12.1.3 The accuracy of isodose plots and graphic displays;
11.12.1.4 The accuracy of the software used to determine radiation source positions from radiographic images; and
11.12.1.5 If the treatment-planning system is different from the treatment-delivery system, the accuracy of electronic transfer of the treatment delivery parameters to the treatment delivery unit from the treatment planning system.
11.12.2 The position indicators in the applicator shall be compared to the actual position of the source or planned dwell positions, as appropriate, at the time of commissioning.
11.12.3 Prior to each patient treatment regimen, the parameters for the treatment shall be evaluated and approved by the authorized user and the Qualified Medical Physicist for correctness through means independent of that used for the determination of the parameters.
11.13 Training.
11.13.1 A registrant shall provide instruction, initially and at least annually, to all individuals who operate the electronic brachytherapy device, as appropriate to the individual's assigned duties, in the operating procedures identified in 11.8. If the interval between patients exceeds one year, retraining of the individuals shall be provided.
11.13.2 In addition to the requirements of 3.3 for therapeutic radiation machine authorized users and 3.4 for Qualified Medical Physicists, these individuals shall also receive device specific instruction initially from the manufacturer, and annually from either the manufacturer or other qualified trainer. The training shall be of a duration recommended by a recognized national professional association with expertise in electronic brachytherapy (when available). In the absence of any training protocol recommended by a national professional association, the manufacturer’s training protocol shall be followed. The training shall include, but nor be limited to:
11.13.2.1 Device-specific radiation safety requirements;
11.13.2.2 Device operation;
11.13.2.3 Clinical use for the types of use approved by the FDA;
11.13.2.4 Emergency procedures, including an emergency drill; and
11.13.2.5 The registrant’s Quality Assurance Program.
11.13.3 A registrant shall retain a record of individuals receiving instruction required by 11.13.1 and 11.13.2 for three (3) years The record shall include a list of the topics covered, the date of the instruction, the name(s) of the attendee(s), and the name(s) of the individual(s) who provided the instruction.
11.14 Mobile Electronic Brachytherapy Service. A registrant providing mobile electronic brachytherapy service shall, as a minimum:
11.14.1 Check all survey instruments before medical use at each address of use or on each day of use, whichever is more restrictive.
11.14.2 Account for the electronic brachytherapy source in the electronic brachytherapy device before departure from the client’s address.
11.14.3 Perform, at each location on each day of use, all of the required quality assurance checks specified 11.11 to assure proper operation of the device.
12.1 A person shall not utilize any device which is designed to electrically generate a source of ionizing radiation to deliver therapeutic radiation dosage, and which is not appropriately regulated under any existing category of therapeutic radiation machine, until:
12.1.1 The applicant or registrant has, at a minimum, provided the Agency with:
12.1.1.1 A detailed description of the device and its intended application(s);
12.1.1.2 Facility design requirements, including shielding and access control;
12.1.1.3 Documentation of appropriate training for authorized user physician(s) and qualified medical physicist(s)
12.1.1.4 Methodology for measurement of dosages to be administered to patients or human research subjects;
12.1.1.5 Documentation regarding calibration, maintenance, and repair of the device, as well as instruments and equipment necessary for radiation safety
12.1.1.6 Radiation safety precautions and instructions; and
12.1.1.7 Other information requested by the Agency in its review of the application; and
12.1.2 The applicant or registrant has received written approval from the Agency to utilize the device in accordance with the regulations and specific conditions the Agency considers necessary for the medical use of the device.
1.1 Basic facility information including: name, telephone number and Agency registration number of the individual responsible for preparation of the shielding plan; name and telephone number of the facility supervisor; and the street address [including room number] of the therapeutic radiation machine facility. The plan should also indicate whether this is a new structure or a modification to existing structure(s).
1.2 All wall, floor, and ceiling areas struck by the useful beam shall have primary barriers.
1.3 Secondary barriers shall be provided in all wall, floor, and ceiling areas not having primary barriers.
2.1 In addition to the requirements listed in Section I above, therapeutic radiation machine facilities which produce only photons with a maximum energy less than or equal to 150 kV shall submit shielding plans which contain, as a minimum, the following additional information:
2.2 Equipment specifications, including the manufacturer and model number of the therapeutic radiation machine, as well as the maximum technique factors;
2.3 Maximum design workload for the facility including total weekly radiation output, [expressed in gray (rad) or air kerma at 1 meter], total beam‑on time per day or week, the average treatment time per patient, along with the anticipated number of patients to be treated per day or week;
2.4 A facility blueprint/drawing indicating: scale [0.25 inch = 1 foot is typical]; direction of North; normal location of the therapeutic radiation machine's radiation port(s); the port's travel and traverse limits; general direction(s) of the useful beam; locations of any windows and doors; and the location of the therapeutic radiation machine control panel. If the control panel is located inside the therapeutic radiation machine treatment room, the location of the operator's booth shall be noted on the plan and the operator's station at the control panel shall be behind a protective barrier sufficient to ensure compliance with Part D.1201 of these regulations;
2.5 The structural composition and thickness or lead/concrete equivalent of all walls, doors, partitions, floor, and ceiling of the room(s) concerned;
2.6 The type of occupancy of all adjacent areas inclusive of space above and below the room(s) concerned. If there is an exterior wall, show distance to the closest area(s) where it is likely that individuals may be present; and
2.7 At least one example calculation which shows the methodology used to determine the amount of shielding required for each physical condition [i.e.: primary and secondary/leakage barriers, restricted and unrestricted areas, entry door(s)] and shielding material in the facility:
2.7.1 If commercial software is used to generate shielding requirements, please also identify the software used and the version/ revision date.
2.7.2 If the software used to generate shielding requirements is not in the open literature, please also submit quality control sample calculations to verify the result obtained with the software.
3.1 In addition to the requirements listed in Section I above, therapeutic radiation machine facilities that produce photons with a maximum energy in excess of 150 kV and/or electrons shall submit shielding plans which contain, as a minimum, the following additional information:
3.2 Equipment specifications including the manufacturer and model number of the therapeutic radiation machine, and gray (rad) at the isocenter and the energy(s) and type(s) of radiation produced [i.e.: photon, electron]. The target to isocenter distance shall be specified;
3.3 Maximum design workload for the facility including total weekly radiation output [expressed in gray (rad) at 1 meter], total beam‑on time per day or week, the average treatment time per patient, along with the anticipated number of patients to be treated per day or week;
3.4 Facility blueprint/drawing [including both floor plan and elevation views] indicating relative orientation of the therapeutic radiation machine, scale [0.25 inch = 1 foot is typical], type(s), thickness and minimum density of shielding material(s), direction of North, the locations and size of all penetrations through each shielding barrier [ceiling, walls and floor], as well as details of the door(s) and maze;
3.5 The structural composition and thickness or concrete equivalent of all walls, doors, partitions, floor, and ceiling of the room(s) concerned;
3.6 The type of occupancy of all adjacent areas inclusive of space above and below the room(s) concerned. If there is an exterior wall, show distance to the closest area(s) where it is likely that individuals may be present;
3.7 Description of all assumptions that were in shielding calculations including, but not limited to, design energy [i.e.: room may be designed for 6 MV unit although only a 4 MV unit is currently proposed], work‑load, presence of integral beam‑stop in unit, occupancy and use(s) of adjacent areas, fraction of time that useful beam will intercept each permanent barrier [walls, floor and ceiling] and "allowed" radiation exposure in both restricted and unrestricted areas; and
3.8 At least one example calculation which shows the methodology used to determine the amount of shielding required for each physical condition [i.e.: primary and secondary/leakage barriers, restricted and unrestricted areas, small angle scatter, entry door(s) and maze] and shielding material in the facility:
3.8.1 If commercial software is used to generate shielding requirements, also identify the software used and the version/ revision date; and
3.8.2 If the software used to generate shielding requirements is not in the open literature, also submit quality control sample calculations to verify the result obtained with the software.
4.1 In addition to the requirements listed in Section III above, therapeutic radiation machine facilities that are capable of operating above 10 MV shall submit shielding plans which contain, as a minimum, the following additional information:
4.2 The structural composition, thickness, minimum density and location of all neutron shielding material;
4.3 Description of all assumptions that were used in neutron shielding calculations including, but not limited to, neutron spectra as a function of energy, neutron fluence rate, absorbed dose and dose equivalent (due to neutrons) in both restricted and unrestricted areas;
4.4 At least one example calculation which shows the methodology used to determine the amount of neutron shielding required for each physical condition [i.e.: restricted and unrestricted areas, entry door(s) and maze] and neutron shielding material utilized in the facility:
4.4.1 If commercial software is used to generate shielding requirements, also identify the software used and the version/ revision date; and
4.4.2 If the software used to generate shielding requirements is not in the open literature, also submit quality control sample calculations to verify the result obtained with the software.
4.5 The method(s) and instrumentation that will be used to verify the adequacy of all neutron shielding installed in the facility.
5.1 NCRP Report 49, "Structural Shielding Design and Evaluation for Medical Use of X Rays and Gamma Rays of Energies Up to 10 MeV" (1976).
5.2 NCRP Report 79, "Neutron Contamination from Medical Electron Accelerators" (1984).
5.3 NCRP Report 144, "Radiation Protection for Particle Accelerator Facilities" (2003).
5.4 NCRP Report 151, “Structural Shielding Design and Evaluation for Megavoltage X- and Gamma-Ray Radiotherapy Facilities. (2006).







